Figure 4. A new model for the activation of cofilin at the leading edge of locomotory protrusions.
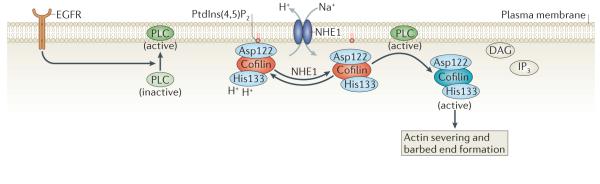
Inactive cofilin is bound to phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) at the plasma membrane through its Asp122 residue. Na+–H+ exchanger 1 (NHE1) can increase the intracellular pH by exchanging intracellular H+ for extracellular Na+. This causes the deprotonation of cofilin at His133, which alters its binding affinity for PtdIns(4,5)P2. An increased pH facilitates phospholipase C (PLC)-mediated hydrolysis of PtdIns(4,5)P2 to diacylglycerol (DAG) and inositol-1,4,5,-trisphosphate (IP3) and the release of cofilin from PtdIns(4,5)P2. Epidermal growth factor receptor (EGFR) stimulates PLC activity, which in turn increases cofilin activation. See BOX 1 for methods describing how to study cofilin mobility as well as cofilin binding to the plasma membrane and F-actin.
