Figure 5.
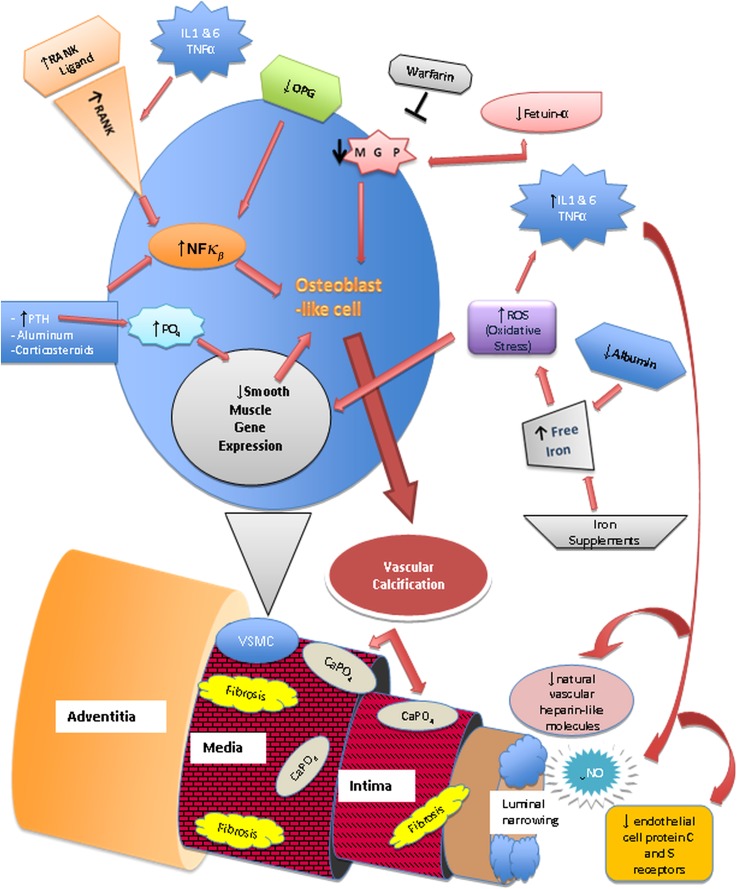
Pathophysiology of calcific uremic arteriolopathy. Nuclear factor κβ (NFκβ), a transcription factor involved in the production of cytokines and inflammatory mediators, the receptor activator of NFκβ (RANK), and its ligand are vital for bone mineral resorption and development (68,73,76). They can be activated by chronic inflammatory states, parathyroid hormone (PTH), aluminum, corticosteroids, free radicals, and infectious agents (73,76). Increased NFκβ activity leads to bone mineral loss and vascular calcification. Osteoprotegerin (OPG) is an antagonist of RANKL. RANK/RANKL and OPG are expressed on endothelial cells, osteoblasts/osteoclasts, and vascular smooth muscle cells (VSMCs) (73,76). Transformation of VSMCs into osteoblast-like cells is initiated by metabolic disturbances (particularly hyperphosphatemia), reactive oxygen species (ROS), and decrease of local vascular calcification inhibitors, such as matrix γ-carboxy-glutamate protein (MGP), a vitamin K-dependent protein (68,76). Fetuin-A, a hepatically synthesized protein that functions systemically to inhibit vascular calcification, is reduced in inflammatory states, renal failure, and calcific uremic arteriolopathy (68,76). Progressive subintimal fibrosis and medial-arteriolar calcification lead to endothelial injury and luminal narrowing. The low-flow rate within the cutaneous vessels combined with luminal narrowing leads to decreased blood flow and may lead to blood stasis (73). Systemic inflammation also causes endothelial dysfunction. It creates a procoagulant state in the narrowed vessels and increases the risk of thrombus formation, which can lead to localized tissue ischemia and necrosis (73,76,78).