Abstract
Immune factors influencing progression to active tuberculosis (TB) remain poorly defined. In this study, we investigated the expression of immunoregulatory cytokines and receptors by using lung bronchoalveolar lavage cells obtained from patients with pulmonary TB, patients with other lung diseases (OLD patients), and healthy volunteers (VOL) by using reverse transcriptase PCR, a transforming growth factor β (TGF-β) bioactivity assay, and an enzyme immunoassay. TB patients were significantly more likely than OLD patients to coexpress TGF-β receptor I (RI) and RII mRNA, as well as interleukin-10 (IL-10) mRNA (thereby indicating the state of active gene transcription in the alveolar cells at harvest). In contrast, gamma interferon (IFN-γ) and IL-2 mRNA was seen in both TB and OLD patients. Likewise, significantly elevated pulmonary steady-state protein levels of IL-10, IFN-γ, and bioactive TGF-β were found in TB patients versus those in OLD patients and VOL. These data suggest that the combined production of the immunosuppressants IL-10 and TGF-β, as well as coexpression of TGF-β RI and RII (required for cellular response to TGF-β), may act to down-modulate host anti-Mycobacterium tuberculosis immunity and thereby allow uncontrolled bacterial replication and overt disease. Delineating the underlying mechanisms of M. tuberculosis-triggered expression of these immune elements may provide a molecular-level understanding of TB immunopathogenesis.
Mycobacterium tuberculosis is the etiologic agent of tuberculosis (TB), and the World Health Organization estimates that one-third of the world's population is infected by M. tuberculosis, with approximately 8 million new TB cases and 2 to 3 million deaths reported annually (16, 54). The majority of individuals are able to restrict infection but thereafter harbor latent M. tuberculosis that can subsequently persist for years to decades without causing illness before later causing reactivation TB (54). Those with immune insufficiency are especially at risk for the development of TB. In fact, the depletion of CD4+ T cells stemming from coinfection with human immunodeficiency virus (HIV) and M. tuberculosis increases the estimated risk of these individuals for the development of active TB (1). It is now clear that coordination and cooperation among the various elements of immunity, especially the macrophages and T lymphocytes, is critical in limiting M. tuberculosis infection (reviewed in references 7 and 18). However, the factors mediating susceptibility and specific resistance to M. tuberculosis remain poorly defined.
T-cell and macrophage functions are predominantly modulated by their local cytokine milieus, and signaling by proinflammatory cytokines is known to be important for the development of counter-M. tuberculosis immune responses (7, 18). For example, the control of M. tuberculosis infection in mice is correlated with the development of Mycobacterium-specific CD4+-T-cell clones that produce the T cell-proliferative and -differentiating signal interleukin-2 (IL-2) as well as the macrophage-activating cytokine gamma interferon (IFN-γ) (4). IL-2 and IFN-γ are also thought to play significant roles in defense against M. tuberculosis infection in humans since elevated mRNA and protein levels are often correlated with better anti-M. tuberculosis immune responses (2, 14, 21). IL-2 serves as the critical regulator of the adaptive T cell-mediated immune response to mycobacterial infection (reviewed in reference 30). IFN-γ mediates its protective effect in mice predominantly by the up-regulation of inducible nitric oxide synthase (NOS2), an enzyme that produces NO and is necessary for the killing of phagocytosed tubercle baccilli (6, 17, 19); unrestricted M. tuberculosis growth is seen in gene knockout mice in which IFN-γ or NOS2 genes have been disrupted (9, 19, 31). Similarly, a natural human mutation of the IFN-γ receptor that renders the receptor functionless has been associated with increased susceptibility to disseminated Mycobacterium infection (29, 36). It has also been reported that IFN-γ-treated human macrophages are able to inhibit and kill M. tuberculosis when in the presence of primed peripheral lymphocytes (3). Although the role of NOS2 products in protection against M. tuberculosis infection in humans remains somewhat controversial, NOS2 transcripts, protein, and activity have been detected in the alveolar macrophages from active-TB patients (37, 52). Moreover, NO from human alveolar macrophages can contribute to the killing of tubercle bacilli in vitro (38, 41). However, despite the above observations, the preferred intracellular niche of M. tuberculosis remains the usually hostile phagolysosome of the alveolar macrophage, and active pulmonary TB is generally accompanied by suppressed T cell-mediated responses to M. tuberculosis antigens (5, 48). Suboptimal cytokine signaling may therefore play a role in the development of TB.
The modulation of the cytokine environment to alter T-cell function and/or prevent macrophage activation through the exploitative induction of immunosuppressive cytokines that counteract the immune response-activating actions of IL-2 and IFN-γ is another possible mechanism by which M. tuberculosis avoids sterilizing immunity. IL-10 and transforming growth factor β (TGF-β) are two such potential deactivators of the immune response in TB. To various degrees, IL-10 and TGF-β inhibit T-cell proliferation and differentiation and the production of IL-2 and IFN-γ as well as antagonize many IFN-γ-mediated actions, including monocyte/macrophage activation and killing of ingested microorganisms (reviewed in references 22 and 34). Sustained secretion of IL-10 and TGF-β has also been associated with the induction of a long-lasting state of hyporesponsiveness (anergy) to specific nonself antigens (53, 55). Bioresponses similar to those induced by IL-10 and/or TGF-β have also been observed in the context of in vitro stimulation of pulmonary-TB patients' peripheral blood mononuclear cells and/or monocytes with M. tuberculosis antigens (5, 25, 40, 43). Likewise, increased levels of IL-10 and TGF-β in sera from TB patients have been reported, as has increased in vitro IL-10 and TGF-β secretion by the peripheral blood mononuclear cells and/or monocytes of TB patients in response to M. tuberculosis antigens (14, 24, 39, 50). In fact, M. tuberculosis can directly induce antigen-presenting cell (APC) IL-10 and TGF-β production (3, 20, 47) and the ability of infected human monocytes/macrophages to restrict growth of M. tuberculosis has correlated inversely with the amount of stimulated IL-10 and TGF-β secretion (3, 26). Therefore, TGF-β and IL-10 appear to support in vitro intrahuman monocyte growth of M. tuberculosis and intersecting studies suggest that this is accomplished in part by inhibiting IFN-γ-induced NO synthesis in activated macrophages (11, 12). Corroborating infection studies of IL-10 knockout and IL-10-overexpressing transgenic mice have further shown that IL-10 is an inhibitor of early mycobacterial clearance and that IL-10 attenuates antimycobacterial immunity (28, 35, 51). These findings led us to question whether the expression of cytokines that oppose immune response activation may have associated roles in human pulmonary TB. We initiated this study using lung cells and lung fluid obtained from patients with active pulmonary TB to evaluate the expression and balance of the pro- and anti-inflammatory mediators.
MATERIALS AND METHODS
Patients, specimens, and study design.
Patients included in this study were from the University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro, a TB referral center serving ∼1 million residents (10% of the population) of greater Rio de Janeiro, Brazil. The incidence of TB in Brazil in the year 2000 was 48.4 per 100,000 persons, and that in Rio de Janeiro was 91.9 per 100,000 persons (23). An additional two patients (R2 and R3) included in this study were from the Pulmonary Service, Bellevue Hospital, New York, N.Y. Patients were referred to the pulmonary services for diagnostic fiber-optic bronchoscopy and bronchoalveolar lavage (BAL) because of abnormal chest X-rays and clinical suspicion of TB and because expectorated sputum was unavailable or negative for acid-fast bacilli (AFB) (see Table 1). A bronchoscopist, not a member of the research team, approved all requests and performed the procedures. Excluded from the study were patients on antituberculosis drugs and those with diabetes, chronic hepatic failure, or chronic renal failure. Once an eligible patient was identified, members of the research team approached the referring physician for authorization to use excess cells from the diagnostic bronchoscopy and BAL. HIV counseling was provided after obtaining the patient's informed consent. HIV type 1-positive patients were excluded. Informed consent was obtained from all patients and volunteers. This study protocol was approved by the institutional review boards of the Federal University of Rio de Janeiro and the Weill Medical College of Cornell University. Bronchoscopy and BAL were performed in compliance with international standards. Briefly, following injection of atropine and light sedation, local anesthetization of upper and lower airways was performed with 2% lidocaine, and a fiber-optic bronchoscope was introduced into the diseased bronchi. Next, 5 to 6 aliquots of sterile saline (20 ml each) were introduced and aspirated sequentially with a minimum of 50% recovery of the injected fluid. The total volume of the BAL fluid recovered was measured, a portion was sent for diagnostic testing, and the remainder was placed in siliconized bottles on ice and was processed for this study.
TABLE 1.
Demographic, clinical, and laboratory data for all subjects in the studya
| Clinical characteristic | Value for:
|
||
|---|---|---|---|
| TB patients (n = 27) | OLD patients (n = 25) | VOL (n = 8) | |
| Mean age ± SE (yr) | 37.0 ± 2.5 | 46.6 ± 3.3 | 34.6 ± 2.7 |
| No. of males (%) | 18 (67) | 18 (69) | 8 (100) |
| No. of smokers (%) | 11 (41) | 13 (50) | 0 (0) |
| Mean duration of symptoms ± SE (wk) | 8.6 ± 4.6 | 5.5 ± 1.5 | |
| No. of subjects (total no. with data available) with: | |||
| Positive tuberculin results | 9 (19) | 6 (24) | 0 (8) |
| <1 Lung lobe involved | 11 (20) | 8 (18) | |
| ≥1 Lung lobe involved | 9 (20) | 10 (18) | |
| Cavitation | 5 (20) | 2 (22) | |
| No. AFB smear positive (%) | 8 (30) | ||
| Mean no. of BAL cells per ml (106) ± SE | 11.4 ± 0.6 | 10.4 ± 0.2 | 12.5 ± 2.3 |
| % Macrophages | 66.6 ± 5.0 | 69.7 ± 5.4 | 87.1 ± 1.5 |
| % Lymphocytes | 9.9 ± 1.1 | 7.9 ± 0.8 | 10.5 ± 1.8 |
| % Neutrophils | 19.5 ± 5.3*,** | 10.2 ± 4.2* | 0.4 ± 0.1** |
| % Eosinophils | 2.1 ± 0.3 | 2.1 ± 0.8 | 1.5 ± 0.4 |
| % Epithelial cells | 1.3 ± 0.3 | 3.3 ± 1.5 | 0.6 ± 0.6 |
The indicated subjects are those from the two phases of the study with TB patients and controls consisting of VOL and patients with OLD. All subjects were HIV type 1 seronegative. No patient had received anti-TB or specific antimicrobial therapy at the time of bronchoscopy with BAL. A TB case was defined by culture identification of M. tuberculosis (n = 19) or by clinical response to anti-TB treatment in cases in which the M. tuberculosis culture was either negative or contaminated (n = 8; one of these patients was AFB smear positive). OLD included neoplasm (n = 7), scarring from presumed healed TB (n = 6), bacterial pneumonitis as defined by clinical cure after a short course of non-anti-TB antibiotics (n = 10), and rheumatoid lung conditions associated with arthritis (n = 2). Only 20 TB and 22 OLD patients had sufficient information in their medical records to provide detailed chest X-ray data on the number of lobes involved and the presence or absence of cavitary lesions. Two of the patients with OLD diagnosed as cancer had lobar infiltrates and pleural effusion. The morphology of BAL cells was analyzed by microscopy of cytospin cells stained by May-Grumwald-Giemsa. Statistically significant differences between cellular constituents in TB and OLD patients were not detected except for the percentage of neutrophils. *, P < 0.05; **, P < 0.05 (Kruskal-Wallis).
BAL cells and fluid.
A known volume of the BAL fluid was filtered through sterile gauze. Cells obtained by centrifugation (500 × g at 4°C for 10 min) were washed and then counted in a Neubauer chamber (>90% were viable as determined by trypan blue exclusion). The pelleted cells were resuspended in 500 μl of RNAStat60 (Cinna/Biotecx, Houston, Tex.), and BAL fluid depleted of cells was stored at −80°C. The collection of patient samples was performed in two phases. In the first phase of the study, cells from BAL fluid were used for reverse transcriptase (RT)-PCR assays of genes for the following products: IL-2, IFN-γ, IL-10, and TGF-β receptor I (RI) and RII. Of the 30 patients from Rio de Janeiro and the 2 TB patients from New York (R2 and R3), 22 patients had sufficient RNA for all the RT-PCR assays and had good-quality RNA as judged from β-actin cDNA amplicons. These included TB patients (n = 16) and patients with other lung diseases (OLD patients; n = 6). In the second phase of the study, the BAL sample supernatants were used to evaluate cytokine protein bioactivities or quantities of TGF-β, IFN-γ, IL-2, and IL-10 in the following groups: TB patients (n = 24), controls with OLD (n = 25), and healthy volunteers (VOL; n = 8). Table 1 summarizes data for all subjects studied in either or both phases of this report: TB patients (n = 27), OLD patients (n = 25), and VOL (n = 8).
Oligonucleotide primers, probes, mRNA, and RT-PCR.
RNA extracted from BAL cells by following the instructions of the RNAStat60 manufacturer (Cinna/Biotecx) had an A260/A280 ratio of >1.9 for each sample. RNA was reverse transcribed into cDNA (GeneAmp RNA-PCR kit; Perkin-Elmer Cetus, Norwalk, Conn.) in a reaction mixture incubated for 30 min at 42°C and heated to 99°C for 5 min and then 4°C for 5 min. PCR was performed with 35 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1.5 min. The amount of input cDNA for cytokine and cytokine receptor PCR was adjusted based on the ratio of the sample's RT-PCR β-actin amplicon densitometry reading to the mean β-actin amplicon densitometry reading of all the samples. The PCR primers used were as follows: IFN-γ, 5′-ATG AAA TAT ACA AGT TAT ATC TTG GCT TT-3′ (sense) and 5′-GAT GCT CTT CGA CCT CGA AAC AGC AT-3′ (antisense); IL-2, 5′-ATG TAC AGG ATG CAA CTC CTG TCT T-3′ (sense) and 5′-GTC AGT GTT GAG ATG ATG CTT TGA C-3′ (antisense); β-actin, 5′-TGA CGG GGT CAC CCA CAC TGT GCC TAT CTA-3′ (sense) and 5′-CTA GAA GCA TTG CGG TGG ACG ATG GAG GG-3′ (antisense); IL-10, 5′-TCT CAA GGG GCT GGG TCA GCT ATC CCA-3′ (sense) and 5′-ATG CCC CAA GCT GAG AAC CAA GAC CCA-3′ (antisense); TGF-β RI, 5′-CGT GCT GAC ATC TAT GCA AT-3′ (sense) and 5′-AGC TGC TCC ATT GGC ATA C-3′ (antisense; these primers annealed to bases 1268 to 1287 and 1519 to 1501, respectively, of the human type I TGF-β receptor gene [GenBank accession no. L11695]); and TGF-β RII, 5′-GAC ATC TCG CTG TAA TGC AGT GG-3′ (sense) and 5′-TAG GGA GCC GTC TTC AGG AAT C-3′ (antisense; these primers annealed to bases 7 to 29 and 325 to 304, respectively, of the human type II TGF-β receptor gene [GenBank accession no. M85079]) (33).
Autoradiography.
Equal portions of each PCR product were subjected to electrophoresis and transferred onto a nylon membrane (Schleicher & Schuell, Keene, N.H.) (37). Membranes were prehybridized in commercial prehybridization solution (Life Technologies, Grand Island, N.Y.). Hybridization in commercial hybridization solution (SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]; Life Technologies) was performed by following the manufacturer's protocol, with 5 × 105 cpm of 32P-labeled probes targeting internal sequences to the primer sets of each ligand or receptor for 12 to 18 h at 42°C. Filters were washed twice with 2× SSC and 0.1% sodium dodecyl sulfate at room temperature for 15 min each, washed once with 0.1× SSC and 0.5% sodium dodecyl sulfate for 20 to 30 min, wrapped in cellophane, and exposed to X-ray film (hyperfilm-MP; Amersham Corporation, Arlington Heights, III.) at −70°C with an intensifier screen.
Cytokine assays.
A biologic assay was used to detect TGF-β (see below), and patient samples with sufficient cell-free BAL fluid remaining were then assayed for the cytokines IL-2, IFN-γ, and IL-10 by enzyme immunoassay (EIA) (see below). BAL fluids depleted of cells were concentrated by centrifugation (3,000 × g for 180 min at 4°C) using Centriplus-10 membrane filter concentration columns (Amicon Inc., Beverly, Mass.). The concentration factor was calculated by dividing the initial volume by the final volume retained by the membrane. Differences in the BAL fluid concentration factors among the three groups were not detected (TB patients, 2.14 ± 0.36; OLD patients, 1.92 ± 0.20; and VOL, 2.20 ± 0.36; P = 0.332; Kruskal-Wallis). By using each sample's concentration factor, the quantitated cytokine values were then converted into values expressing the actual levels in the saline-diluted BAL fluid at harvest. TGF-β activity was determined by a bioassay that responds to TGF-β1, TGF-β2, and TGF-β3 by inhibiting proliferation (as described in reference 3). In brief, concentrated cell-free BAL supernatants from TB and control patients were tested for proliferation-inhibitory activity on Mv1Lu mink lung cells (ATCC CCL-64) by assessing [3H]thymidine incorporation (200 μl at 0.1 μCi/well). The amount of TGF-β was extrapolated from a standard curve by using recombinant human TGF-β1, and the specific TGF-β activity was established in parallel using antisera with neutralizing activity against TGF-β1, TGF-β2, and TGF-β3 (33). Specific TGF-β activity was calculated as the difference in results between the no-antisera and neutralizing-antisera conditions. Other cytokine levels were determined by commercial EIA as per the manufacturer's protocol for IL-2 and IL-10 (immunoassay kit; Immunotech-Coulter Corporation, Miami, Fla.) as well as IFN-γ (Cytoscreen immunoassay kit; BioSource International, Camarillo, Calif.). Statistical significance was defined as a P value of <0.05.
RESULTS
Expression of mRNA for cytokine and cytokine receptor genes in BAL cells.
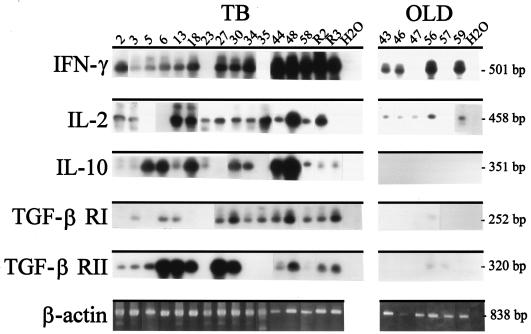
To examine the immunological parameters associated with M. tuberculosis infection, TB patients and control OLD patients were tested for the expression of IL-2, IFN-γ, and IL-10 mRNA, as well as TGF-β RI and RII mRNA, by RT-PCR (Fig. 1). The expression of IL-2 and IFN-γ was seen in all 16 TB patients and in 4 and 5, respectively, of the 6 OLD patients. In contrast, IL-10 was selectively detected in 14 of 16 TB patients and in none of the 6 OLD patients (P < 0.05; Fischer's exact test). Moreover, the majority of TB patients (10 of 16) coexpressed TGF-β RI and RII while only one of six OLD patients had a faint TGF-β RI and RII amplicon (P < 0.05; Fisher's exact test). The BAL cells were predominantly macrophages, and with the exception of the neutrophils, the cellular constituents were similar between patients with TB and those with OLD (Table 1). Included in this analysis were 13 TB patients for whom the evaluation of NOS2 transcripts was previously reported (n = 11) (37) or performed (n = 2; data not shown). Of these 13 TB cases, only 3 patients had no NOS2 transcripts detectable by RT-PCR (these 3 TB cases also had strong expression of IL-10 as well as TGF-β RI and/or RII; patient numbers are given in the legend to Fig. 1). Therefore, the potential NOS2-mediated mechanism of mycobacterial control appeared to remain intact for most of the evaluated patients.
FIG. 1.
The BAL cells of TB patients express transcripts for IL-2, IFN-γ, and IL-10, as well as TGF-β RI and RII. Illustrated are the autoradiographic blot analyses of RT-PCR products for IL-2, IFN-γ, IL-10, TGF-β RI, and TGF-β RII, as well as the ethidium bromide-stained RT-PCR β-actin amplicons, from BAL cells obtained from active-TB patients (TB; n = 16) or control patients with OLD (OLD; n = 6). The amounts of input cDNA from cytokine and cytokine receptor RT-PCR were each normalized to the ratio of the sample's β-actin amplicon density to the mean β-actin amplicon density (see Materials and Methods). Amplicons were electrophoresed, transferred onto a nylon membrane, hybridized with specific 32P-labeled probes, and exposed to X-ray film. The number above each lane corresponds to a patient code. The expected band sizes are given at right. Water (H2O) was loaded as the negative control. Thirteen patients were studied for NOS2 by RT-PCR and Southern analysis, and all except three patients (numbers 5, 6, and 34) had NOS2 transcripts detected. NOS2 expression in 11 of these patients (numbers 2, 3, 5, 6, 27, 30, 34, 35, 44, R2, and R3) was previously reported (37). Patients 13 and 18 were also positive for NOS2 (data not shown). Patients R2 and R3 were from New York and all others were from Brazil. Fourteen patients with TB (numbers 2, 3, 5, 6, 13, 18, 23, 27, 30, 34, 35, 44, 48, and 58) and six patients with OLD (numbers 43, 46, 47, 56, 57, and 59) had both RT-PCR assays and TGF-β bioassays performed (see the text). IL-10 was also assayed by both RT-PCR and EIA for two TB patients (numbers 44 and 58) and two patients with OLD (numbers 46 and 59) (see the text). This image was created using Adobe PhotoDeluxe Home Edition 3.1.
Evaluation of BAL cytokine levels.
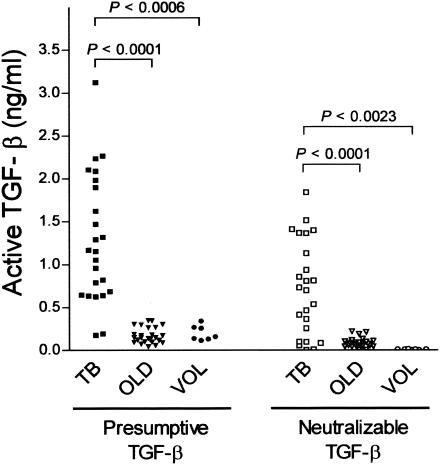
We next assayed the concentrated BAL fluid for active TGF-β (as opposed to the latent precursor form) by utilizing a biological assay in which the growth of sensitive Mv1Lu cells is inhibited by functional TGF-β. Significantly higher amounts of active TGF-β and neutralizable TGF-β were observed in TB patients than in either OLD patients (P < 0.0001) or VOL (P < 0.0018; unpaired two-tailed t test) (Fig. 2).
FIG. 2.
TGF-β activity is elevated in the BAL fluids of TB patients. Active and neutralizable TGF-β activity from the lavage fluids (concentrated by molecular sieve centrifugation) of TB patients (TB; n = 24), controls with OLD (OLD; n = 25), and VOL (n = 8) was detected by a bioassay that evaluates TGF-β-induced proliferation inhibition of the Mv1Lu mink lung epithelial cell line. The amount of TGF-β was extrapolated from a standard curve by using recombinant human TGF-β1, and the specific TGF-β activity was established in parallel by using antisera with neutralizing activity against TGF-β1, TGF-β2, and TGF-β3. Specific TGF-β activity was calculated as the difference between results for the no-antisera and the neutralizing antisera conditions. The results of statistical analyses of the data by the unpaired two-tailed t test are shown. TGF-β levels determined by bioassay were comparatively examined within the TB group by three separate categorical segregations: cavitary versus noncavitary disease, extensive (two or more lobes affected) versus limited (one lobe affected) disease, and TB diagnosis based upon AFB culture positivity versus diagnosis based upon clinical criteria (as given in Table 1). No differences were seen in protein levels of TGF-β among the segregated TB subgroupings.
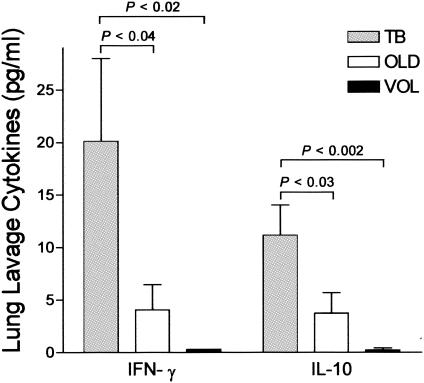
Samples with sufficient BAL fluid remaining from the previous experiments were then assayed for the cytokines IL-10, IFN-γ, and IL-2 by EIA (Fig. 3). Results were obtained for each cytokine from all donors. TB patients (n = 10) had statistically significantly higher levels of IL-10 than OLD patients (n = 10) and VOL (n = 8) (P < 0.02; unpaired one-tailed t test). Interestingly, TB patients also had significantly elevated IFN-γ levels compared to OLD patients (n = 10) and VOL (n = 8) (P < 0.01; unpaired one-tailed t test). IL-2 levels varied widely and were not statistically significantly different among the patient groups (data not shown). However, whether these data represent a real biological phenomenon or whether they have been influenced by the short dimer half-life of IL-2 is not known (10).
FIG. 3.
Levels of IFN-γ and IL-10 are elevated in the BAL fluids from TB patients. Lung lavage fluids from TB patients (TB; n = 10), controls with OLD (OLD; n = 10), and VOL (n = 8) were assayed for both IFN-γ and IL-10 by commercial EIA. The standard-error bars, as well as the results of statistical analyses of the data by the unpaired one-tailed t test, are shown.
To address the question of whether the cytokine increases seen in TB may simply reflect active inflammation in the lungs versus no inflammation, the OLD group was segregated into two groups, those with inflammation (bacterial pneumonitis, presumed healed TB, and rheumatoid lung conditions) and those with cancer, and analyzed by using active neutralizable TGF-β. TB patients had significantly higher median TGF-β levels (549 pg/ml [25th percentile, 410 pg/ml; 75th percentile, 701 pg/ml]) than OLD patients with inflammation (52 pg/ml [25th percentile, 7 pg/ml; 75th percentile, 105 pg/ml]) or cancer (103 pg/ml [25th percentile, 27 pg/ml; 75th percentile, 153 pg/ml]) (P < 0.0001; Mann-Whitney rank sum test). The difference in TGF-β levels between the cancer and inflammation OLD subgroups was not statistically significant (P > 0.05; Dunn's posttest following Kruskal-Wallis), while the differences in TGF-β levels between TB patients and those in the OLD subgroups remained statistically significant (P < 0.05; Dunn's posttest following Kruskal-Wallis). We further examined 14 patients with TB and 6 patients with OLD who had both RT-PCR and the TGF-β bioassay performed (see the legend to Fig. 2). Higher neutralizable TGF-β levels were generally observed in these TB patients than in the OLD patients (463 pg/ml [25th percentile, 385 pg/ml; 75th percentile, 694 pg/ml] versus 45 pg/ml [25th percentile, 7 pg/ml; 75th percentile, 108 pg/ml]; P < 0.001; Mann-Whitney rank sum test). In particular, eight TB patients had high TGF-β RI and RII coexpression and high levels of TGF-β ligand (437.7 ± 61.6 pg of neutralizable TGF-β/ml [mean ± standard error]) while the only OLD patient (number 57) who had faint TGF-β RI and RII coexpression also had a similarly low level of TGF-β ligand (6.8 pg/ml). For IL-10, samples from two TB patients and two patients with OLD were subjected to both RT-PCR and protein cytokine assays; IL-10 mRNA and protein (3.75 ± 1.4 pg/ml) were present in the TB patients but not in the OLD patients as determined by RT-PCR and EIA (see the legend to Fig. 3). Therefore, the differences in levels of cytokines and cytokine receptors detected between TB and OLD patients were unlikely due to differences in noninflammatory diseases in OLD patients. Finally, based upon the clinical characteristics presented in Table 1, TB patients had relatively limited to moderate disease as shown by the number of lobes involved, cavitation, and the rate of AFB smear positivity. These clinical features in our study reinforce the hypothesis that down-modulatory cytokines and receptors were triggered by M. tuberculosis and are reflective of the pathobiology of active TB and not solely due to immune collapse in late or severe disease.
DISCUSSION
Susceptibility to TB is likely mediated by a combination of multiple microbial and host factors that have yet to be fully identified and understood. For the most part, studies addressing immunomodulating cytokine production in the TB-diseased lung have relied on murine models (9, 35) and in vitro M. tuberculosis antigen rechallenge of ex vivo human BAL cells from TB patients and their contacts (32, 45) or BAL cells from patients with tuberculous pleurisy (a self-limiting disease) (2, 44). Few studies have comprehensively evaluated the unadulterated cytokine profiles in the lungs of active-TB patients. In evaluating BAL samples and unstimulated BAL cells, our investigations focused on host factors in the lung, the primary site of M. tuberculosis infection. As a result, these data depict the ongoing immunological situation in the human TB-diseased lung and may be more relevant than measurements of TB immunity commonly made from the circulating compartment alone. Moreover, by analyzing several immune parameters, this study provides a broad and unique perspective of the potential correlates of suboptimal immunity present at the tissue level in TB patients.
At first, the results of this study may seem contradictory. Unlike those of controls, BAL cells from the majority of TB patients transcribed mRNA for IFN-γ, IL-2, and NOS2 (data obtained by cross-referencing this study with reference 18). Furthermore, a significant proportion of TB patients expressed measurable IFN-γ in their BAL fluids. Combined, these data indicate a degree of pulmonary immune response activation in these patients and are supported by similar observations from other laboratories (42, 46). However, these elements were coincident with several anti-inflammatory mediators. In BAL cells from a majority of the TB patients, mRNA for IL-10, as well as for TGF-β RI and RII, was transcribed. In addition, the BAL fluids from a significant number of the tested TB cases also contained elevated IL-10 levels and bioactive TGF-β. The expression of both TGF-β RI and RII is required for proper cell signaling in response to TGF-β ligand (33). As such, the coincidently elevated expression of both bioactive TGF-β (as opposed to the inactive or pro-TGF-β detected by EIA) and its two receptors infers that the activity of this immunosuppressing agent is heightened in the TB-diseased lung and indicates a new correlate of TB pathogenesis. Variables such as patient genetic heterogeneity, as well as chronological differences with respect to hospital presentation and sample acquisition within each patient's disease course, cannot be controlled and so may account for the patient-to-patient variability that was seen. It should also be noted that the amounts of each cytokine detected in the BAL supernatants likely represent steady-state protein levels but that cytokine mRNA data report upon the active state of gene transcription in the alveolar cells at harvest. The finding that many of the patients with OLD had no IL-10 mRNA while they did have the IL-10 protein suggests that IL-10 induction in TB is more sustained than that in OLD. However, this remains to be substantiated by longitudinal studies. Importantly, the levels of the cytokines detected in BAL fluid at harvest are an underestimate since the lung fluid was significantly diluted by up to 1,000-fold due to the instillation of 50 to 100 ml of saline into the sampled area, thereby suggesting probable lung tissue cytokine levels in the low nanograms-per-milliliter range for IL-10 and IFN-γ as well as the micrograms-per-milliliter range for TGF-β when adjusted. Furthermore, the amounts detected in this study were those of cytokines released into the alveolar space, and so both cytokines are likely present at much higher levels at the infection foci. Given that a previous study has shown that levels of IL-10 and TGF-β as low as 10 ng/ml are sufficient to suppress T-cell responses in vitro (40), the detected levels of IL-10 and TGF-β, when adjusted for the instilled saline dilution, likely reflect biologically relevant levels for effecting immunosuppression. Since the immunological response of TB patients is, by definition, suboptimally oriented, our data therefore suggest that it is not the lack of immune response-activating signals that is the root cause of the development of active TB but rather the balance of immune response-down-modulating signals at the site of disease that may be the predominating influence.
Several lines of evidence support the above hypothesis. For instance, IL-10 and TGF-β are central to models of normal immune suppression in ocular immune privilege and mucosal tolerance to innocuous antigens (15, 53). In relation to TB, IL-10-secreting T regulatory 1-like cells have been implicated in the T-cell anergy of TB patients to M. tuberculosis antigens and elevated intrapulmonary production of TGF-β has been observed in lung granuloma macrophages of TB patients by immunostaining (5, 47). Data have recently been reported for a separate cohort of TB patients which positively correlate levels of IL-10 in lung sputum with levels of the M. tuberculosis CFP32 protein (27). These results link IL-10 with increasing bacterial burden (as indicated by the measured CFP32 amounts) and thereby associate IL-10 with the failure in immunity that resulted in TB for this set of patients. Elevated TGF-β levels, as determined by immunohistochemical staining, have also been observed in tissue sections of granulomatous lung lesions from two TB patients (47). That TGF-β and IL-10 may be at the root of active TB is supported by several other additional observations. For instance, TGF-β and IL-10 are known to directly inhibit T-cell activity in response to M. tuberculosis antigens, putatively by suppressing T-cell IL-2 production and/or by depressing IL-2 receptor expression (8, 13, 48); IL-10 and TGF-β also promote T-cell anergy in TB possibly by down-regulating the cell surface expression of the costimulatory and antigen-presenting molecules on M. tuberculosis-infected monocytes (43); finally, IL-10 and TGF-β interfere with the positive feedback loop between APC-derived IL-12 and T cell-produced IFN-γ upon M. tuberculosis stimulation of APCs (20, 21, 49).
The exact mechanism(s) directing lung production of IL-10 and TGF-β in TB is unresolved but may be due to direct stimulation by M. tuberculosis and/or come as a result of the normal dampening of immune responses preceding healing and fibrosis of reactive foci. In any case, the available data support a model for TB in which local IL-10 and TGF-β promote a cytokine microenvironment wherein resident and/or newly recruited immune cells become refractory to appropriate activating signals. Perhaps it is the eventual predomination of IL-10 and TGF-β inhibitory actions that renders immune responses ineffectual for adequate control of tubercle bacilli growth and precipitates the progression to overt disease. Therefore, means of blocking the inhibitory cytokine pathways may improve the outcome of therapeutic interventions for TB. Conversely, the measurement of pulmonary immunosuppressive cytokines may be a useful predictor for the development of TB or a marker for the effectiveness of pharmacologic treatment. Delineating host and M. tuberculosis factors that modulate IL-10, TGF-β, and TGF-β receptor expression may improve our molecular-level understanding of immunopathogenesis in TB.
Acknowledgments
We thank Alice Haffner for statistical analyses and Cesonia Martinusso, Patricia Lago, and Vera Flores for technical assistance.
Financial support was received from the National Institutes of Health (AI-39606 and HL61960 [J.L.H.]; PO1-162821, R37-AI-22624, D43-TW00018, and D43-TW00919 [W.D.J.]; and AG12712 [T.A.M.]) and from the Brazilian Ministry of Health (024/94 DST/AIDS), Fundação Universitária José Bonifácio/FUJB, the Brazilian Research Council/CNPq, and the Brazilian Research Council/World Bank Millennium Institute of Science (M.G.B.-A., A.L.K., and J.R.L.E.S.). M.G.B.-A., L.C.O.L., A.L.K., and J.R.L.E.S. were also supported by Fogarty International Training grants (D43-TW00018 and D43-TW00919).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Barnes, P. F., A. B. Bloch, P. T. Davidson, and D. E. Snider. 1991. Tuberculosis in patients with HIV infection. N. Engl. J. Med. 324:1644-1650. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonecini-Almeida, M. G., S. Chitale, I. Boutsikakis, J. Geng, H. Doo, S. He, and J. L. Ho. 1998. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-gamma and primed lymphocytes. J. Immunol. 160:4490-4499. [PubMed] [Google Scholar]
- 4.Boom, W. H., R. N. Huson, R. A. Young, J. R. David, and W. F. Pissens. 1987. In vivo and in vitro characterization of murine T-cell clones reactive to Mycobacterium tuberculosis. Infect. Immun. 55:2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boussiotis, V. A., E. Y. Tsai, E. J. Yunis, S. Thim, J. C. Delgado, C. C. Dascher, A. Berezovskaya, D. Rousset, J. M. Reynes, and A. E. Goldfeld. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Investig. 105:1317-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, H. L., and S. H. Kaufmann. 2001. The many faces of host responses to tuberculosis. Immunology 103:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook, G., J. D. Campbell, C. E. Carr, K. S. Boyd, and I. M. Franklin. 1999. Transforming growth factor beta from multiple myeloma cells inhibits proliferation and IL-2 responsiveness in T lymphocytes. J. Leukoc. Biol. 66:981-988. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russel, and I. M. Orme. 1993. Disseminated tuberculosis in interferon-gamma gene disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtney, L. P., J. L. Phelps, and L. M. Karavodin. 1994. An anti-IL-2 antibody increases serum half-life and improves anti-tumor efficacy of human recombinant interleukin-2. Immunopharmacology 28:223-232. [DOI] [PubMed] [Google Scholar]
- 11.Cunha, F. Q., S. Moncada, and F. Y. Liew. 1992. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem. Biophys. Res. Commun. 182:1155-1159. [DOI] [PubMed] [Google Scholar]
- 12.Ding, A., C. F. Nathan, J. Graycar, R. Derynck, D. J. Stuehr, and S. Srimal. 1990. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J. Immunol. 145:940-944. [PubMed] [Google Scholar]
- 13.Ding, L., and E. M. Shevach. 1992. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J. Immunol. 148:3133-3139. [PubMed] [Google Scholar]
- 14.Dlugovitzky, D., A. Torres-Morales, L. Rateni, M. A. Farroni, C. Largacha, O. Molteni, and O. Bottasso. 1997. Circulating profile of Th1 and Th2 cytokines in tuberculosis patients with different degrees of pulmonary involvement. FEMS Immunol. Med. Microbiol. 18:203-207. [DOI] [PubMed] [Google Scholar]
- 15.D'Orazio, T. J., and J. Y. Niederkorn. 1998. A novel role for TGF-beta and IL-10 in the induction of immune privilege. J. Immunol. 160:2089-2098. [PubMed] [Google Scholar]
- 16.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 17.Flesch, I., and S. H. Kaufmann. 1987. Mycobacterial growth inhibition by IFN-γ-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J. Immunol. 138:408-413. [PubMed] [Google Scholar]
- 18.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 19.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulton, S. A., J. V. Cross, Z. T. Toossi, and W. H. Boom. 1998. Regulation of interleukin-12 by interleukin-10, transforming growth factor-beta, tumor necrosis factor-alpha, and interferon-gamma in human monocytes infected with Mycobacterium tuberculosis H37Ra. J. Infect. Dis. 178:1105-1114. [DOI] [PubMed] [Google Scholar]
- 21.Gong, J. H., M. Zhang, R. L. Modlin, P. S. Linsley, D. Iver, Y. Lin, and P. F. Barnes. 1996. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect. Immun. 64:913-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorelik, L., and R. A. Flavell. 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2:46-53. [DOI] [PubMed] [Google Scholar]
- 23.Hijjar, M. A., M. J. Procopio Ribeiro de Oliveira, and G. M. Teixeira. 2001. A tuberculose no Brasil e no mundo. Bol. Pneumol. Sanitária 9:9-16. [Google Scholar]
- 24.Hirsch, C. S., J. J. Ellner, R. Blinkhorn, and Z. Toossi. 1997. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc. Natl. Acad. Sci. USA 94:3926-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch, C. S., R. Hussain, Z. Toossi, G. Dawood, F. Shahid, and J. J. Ellner. 1996. Cross-modulation by transforming growth factor-β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon-γ production. Proc. Natl. Acad. Sci. USA 93:3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch, C. S., T. Yoneda, L. E. Averill, J. J. Ellner, and Z. Toossi. 1994. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-β1. J. Infect. Dis. 170:1229-1237. [DOI] [PubMed] [Google Scholar]
- 27.Huard, R. C., S. Chitale, M. Leung, L. C. de Oliveira Lazzarini, H. Zhu, E. Shashkima, S. Laal, M. Conde, A. L. Kritski, J. T. Belisle, B. N. Kreiswirth, J. R. Lapa e Silva, and J. L. Ho. 2003. The Mycobacterium tuberculosis complex-restricted gene cfp32 encodes an expressed protein that is detectable in tuberculosis patients and is positively correlated with pulmonary interleukin-10. Infect. Immun. 71:6871-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs, M., N. Brown, N. Allie, R. Gulert, and B. Ryffel. 2000. Increased resistance to mycobacterial infection in the absence of interleukin-10. Immunology 100:494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jouanguy, E., S. Lamhamedi-Cherradi, F. Altare, M. C. Fondaneche, D. Tuerlinckx, S. Blanche, J. F. Emile, J. L. Gaillard, R. Schreiber, M. Levin, A. Fischer, C. Hivroz, and J. L. Casanova. 1997. Partial interferon-gamma receptor 1 deficiency in child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J. Clin. Investig. 100:2658-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan, G., and V. H. Freedman. 1996. The role of cytokines in the immune response to tuberculosis. Res. Immunol. 147:565-572. [DOI] [PubMed] [Google Scholar]
- 31.MacMicking, J. D., R. J. North, R. La Course, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda, J., N. Ueki, T. Ohkawa, N. Iwahashi, T. Nakano, T. Hada, and K. Higashino. 1993. Local production and localization of transforming growth factor-beta in tuberculous pleurisy. Clin. Exp. Immunol. 92:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaffrey, T. A., S. Consigli, B. Du, D. J. Falcone, T. A. Sanborn, A. M. Spokojny, and H. L. Bush, Jr. 1995. Decreased type II/type I TGF-β receptor ratio in cells derived from human atherosclerotic lesions. J. Clin. Investig. 96:2667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 35.Murray, P. J., and R. A. Young. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 67:3087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylovic, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-γ gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1948. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson, S., M. G. Bonecini-Almeida, J. R. Lapa e Silva, C. Nathan, Q. W. Xie, R. Mumford, J. R. Weidner, J. Calaycay, J. Geng, N. Boechat, C. Linhares, W. Rom, and J. L. Ho. 1996. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J. Exp. Med. 183:2293-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nozaki, Y., Y. Hasegawa, S. Ichiyama, I. Nakashima, and K. Shimokata. 1997. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect. Immun. 65:3644-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olobo, J. O., M. Geletu, A. Demissie, T. Eguale, K. Hiwot, G. Aderaye, and S. Britton. 2001. Circulating TNF-alpha, TGF-beta, and IL-10 in tuberculosis patients and healthy contacts. Scand. J. Immunol. 53:85-91. [DOI] [PubMed] [Google Scholar]
- 40.Othieno, C., C. S. Hirsch, B. D. Hamilton, K. Wilkinson, J. J. Ellner, and Z. Toossi. 1999. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta and interleukin-10. Infect. Immun. 67:5730-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rich, E. A., M. Torres, E. Sada, C. K. Finegan, B. D. Hamilton, and Z. Toossi. 1997. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber. Lung Dis. 78:247-255. [DOI] [PubMed] [Google Scholar]
- 42.Robinson, D. S., S. Ying, I. K. Taylor, A. Wangoo, D. M. Mitchell, A. B. Kay, Q. Hamid, and R. J. Shaw. 1994. Evidence of a Th1-like bronchoalveolar T-cell subset and predominance of interferon-gamma gene activation in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 149:989-993. [DOI] [PubMed] [Google Scholar]
- 43.Rojas, R. E., K. N. Balaji, A. Subramanian, and W. H. Boom. 1999. Regulation of human CD4+ αβ T-cell-receptor-positive (TCR+) and γδ TCR+ T-cell responses to Mycobacterium tuberculosis by interleukin-10 and transforming growth factor β. Infect. Immun. 67:6461-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwander, S. K., M. Torres, C. Carranza, D. Escobedo, M. Tary-Lehmann, P. Anderson, Z. Toossi, J. J. Ellner, E. A. Rich, and E. Sada. 2000. Pulmonary mononuclear cell responses to antigens of Mycobacterium tuberculosis in healthy household contacts of patients with active tuberculosis and healthy controls from the community. J. Immunol. 165:1479-1485. [DOI] [PubMed] [Google Scholar]
- 45.Schwander, S. K., M. Torres, E. Sada, C. Carranza, E. Ramos, M. Tary-Lehmann, R. S. Wallis, J. Sierra, and E. A. Rich. 1998. Enhanced responses to Mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J. Infect. Dis. 178:1434-1445. [DOI] [PubMed] [Google Scholar]
- 46.Taha, R. A., T. C. Kotsimbos, Y. L. Song, M. Menzies, and Q. Hamid. 1997. IFN-γ and IL-12 are increased in active compared with inactive tuberculosis. Am. J. Respir. Crit. Care Med. 155:1135-1139. [DOI] [PubMed] [Google Scholar]
- 47.Toossi, Z., P. Gogate, H. Shiratshuchi, T. Young, and J. J. Ellner. 1995. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculosis granulomatous lung lesions. J. Immunol. 154:465-472. [PubMed] [Google Scholar]
- 48.Toossi, Z., M. E. Kleinhertz, and J. J. Ellner. 1986. Defective IL-2 production and responsiveness in human tuberculosis. J. Exp. Med. 163:1162-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toossi, Z., M. Mincek, E. Seeholtzer, S. A. Fulton, B. D. Hamilton, and C. S. Hirsch. 1997. Modulation of IL-12 by transforming growth factor-beta (TGF-beta) in Mycobacterium tuberculosis-infected mononuclear phagocytes and in patients with active tuberculosis. J. Clin. Lab. Immunol. 49:59-75. [PubMed] [Google Scholar]
- 50.Torres, M., T. Herrera, H. Villareal, E. A. Rich, and E. Sada. 1998. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect. Immun. 66:176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner, J., M. Gonzalez-Juarrero, D. L. Ellis, R. J. Basaraba, A. Kipnis, I. M. Orme, and A. M. Cooper. 2002. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J. Immunol. 169:6343-6351. [DOI] [PubMed] [Google Scholar]
- 52.Wang, C. H., C. Y. Liu, H. C. Lin, C. T. Yu, K. F. Chung, and H. P. Kuo. 1998. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur. Respir. J. 11:809-815. [DOI] [PubMed] [Google Scholar]
- 53.Weiner, H. L. 2001. The mucosal milieu creates tolerogenic dendritic cells and T(R)1 and T(H)3 regulatory cells. Nat. Immunol. 2:671-672. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. 1996. Report of the tuberculosis epidemic. World Health Organization, Geneva, Switzerland.
- 55.Zeller, J. C., A. Panoskaltsis-Mortari, W. J. Murphy, F. W. Ruscetti, S. Narula, M. G. Roncarolo, and B. R. Blazar. 1999. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J. Immunol. 163:3684-3691. [PubMed] [Google Scholar]