Fig. 5.
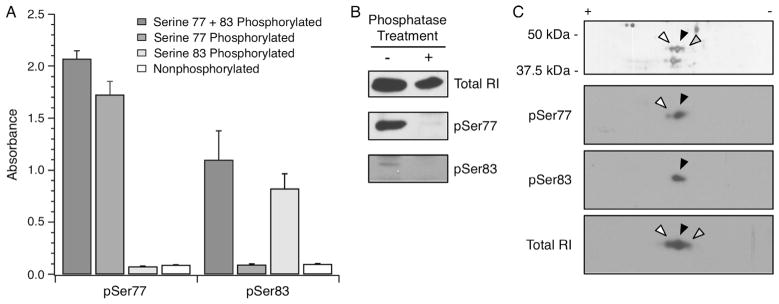
RIα phosphospecific antibody validation. Two rabbit monoclonal antibodies specific for Ser77 or Ser83 phosphorylated RIα were validated. (A) The sequence CAGCTRTDS77REDEIS83PPPNP was synthesized into four peptides to yield non-phosphorylated, Ser77 or Ser83 singly phosphorylated, and Ser77/83 doubly phosphorylated peptides. The culture supernatant from the pSer77 and pSer83 hybridomas were used in ELISA (65) and exhibited high specificity for the intended phosphorylated peptides. (B) Rat myocardial homogenates were left untreated or treated with alkaline phosphatase, followed by blotting with total RI, pSer77 or pSer83 antibodies. A net loss of signal for the Ser77 and Ser83 phosphorylated RIα was observed following alkaline phosphatase treatment. (C) A human myocardium homogenate enriched by 8-AEA-cAMP affinity chromatography for RIα was resolved by 2D SDS–PAGE. The white, black and grey arrowheads represent successively basic spots verified to be RIα by mass spectrometry (data not shown). 2D Western blotting of the same sample showed that the pSer77 and pSer83 antibodies identified the relatively acidic isoelectric variants, whereas the antibody against total RI identified all three isoelectric variants. The spots are aligned with arrowheads in accordance with the accompanying silver stained gel.
