Abstract
Introduction
Morphine is the most effective pain-relieving drug, but it can cause unwanted side effects. Direct neuraxial administration of morphine to spinal cord not only can provide effective, reliable pain relief but also can prevent the development of supraspinal side effects. However, repeated neuraxial administration of morphine may still lead to morphine tolerance.
Methods
To better understand the mechanism that causes morphine tolerance, we induced tolerance in rats at the spinal cord level by giving them twice-daily injections of morphine (20 µg/10 µL) for 4 days. We confirmed tolerance by measuring paw withdrawal latencies and maximal possible analgesic effect of morphine on day 5. We then carried out phosphoproteomic analysis to investigate the global phosphorylation of spinal proteins associated with morphine tolerance. Finally, pull-down assays were used to identify phosphorylated types and sites of 14-3-3 proteins, and bioinformatics was applied to predict biological networks impacted by the morphine-regulated proteins.
Results
Our proteomics data showed that repeated morphine treatment altered phosphorylation of 10 proteins in the spinal cord. Pull-down assays identified 2 serine/threonine phosphorylated sites in 14-3-3 proteins. Bioinformatics further revealed that morphine impacted on cytoskeletal reorganization, neuroplasticity, protein folding and modulation, signal transduction and biomolecular metabolism.
Conclusions
Repeated morphine administration may affect multiple biological networks by altering protein phosphorylation. These data may provide insight into the mechanism that underlies the development of morphine tolerance.
Introduction
Morphine is primarily used to treat severe pain caused by acute injuries and chronic diseases. However, systematic administration of morphine can cause many side effects, including impairment of mental and physical functions, psychological dependence, addiction, and tolerance [1], [2]. Since most of the side effects of morphine occur in the supraspinal regions of the central nervous system (CNS), direct neuraxial administration of morphine to act on spinal cord can prevent the supraspinal side effects and provide effective pain relief [2]. However, repeated neuraxial administration of morphine can still lead to tolerance, which is characterized by loss of analgesic effect of the initial effective dose [3]. Understanding the biomolecular changes associated with repeated neuraxial administration of morphine would be helpful for preventing the development of morphine tolerance.
An animal model in which morphine is repeatedly injected into the spinal cord has been used to mimic the direct neuraxial administration of morphine in patients and to study morphine tolerance at the spinal cord level [4], [5]. Morphine tolerance induced by systematic administration is abolished in spinalized animals [6], supporting the premise that morphine tolerance occurs mainly at the spinal cord level rather than at other CNS regions. Thus, the spinal cord is the key target of morphine tolerance. Analysis of molecular events in the spinal cord in an animal model of morphine tolerance would provide a better understanding of the mechanism of this illness [7].
The development of morphine tolerance is thought to be associated with dysregulated phosphorylation of proteins for two reasons. First, morphine exerts its pharmacologic effects by acting at opioid receptors [8], which transduce signals and modulate protein activity via protein phosphorylation. Disturbance of the pharmacologic effects and signal transduction of morphine have been suggested to cause side effects of morphine [9], [10]. Second, studies of cultured cells and brain tissue have shown that morphine and other opioid agonists can affect phosphorylation of certain proteins [11], [12]. We hypothesize that a specific set of phosphoproteins is likely to be involved in the pathogenesis of morphine tolerance. However, the set of phosphoproteins, i.e. the morphine tolerance-related phosphoproteome, has never been explored, especially in the spinal region.
Phosphoproteomics is used to study the phosphorylation of many proteins (the phosphoproteome), rather than individual proteins in a biological sample [13]. Bioinformatics uses computational algorisms to ascertain the physiologic impact of proteins at the systems level [14]. Both approaches can be used to identify proteins whose roles in a disorder have not been established by other traditional methods. To the best of our knowledge, however, these two approaches have never been used to explore the state of phosphoproteins and their biological networks in spinal cord as they relate to development of morphine tolerance. In this study, we used phosphoproteomics and bioinformatics analysis of spinal cord proteins in rats with morphine tolerance to evaluate the impact of morphine on spinal cord in terms of protein phosphorylation, and to better understand the pathophysiologic mechanism that underlies morphine tolerance.
Materials and Methods
Animals and drug treatment
All animal experiments were carried out with the approval of the Institutional Animal Care and Use Committee at the National Defense Medical Center, Taiwan, and were consistent with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain.
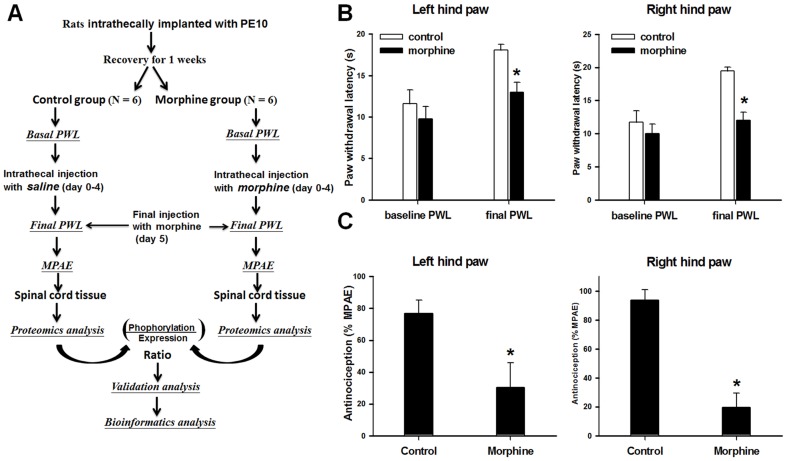
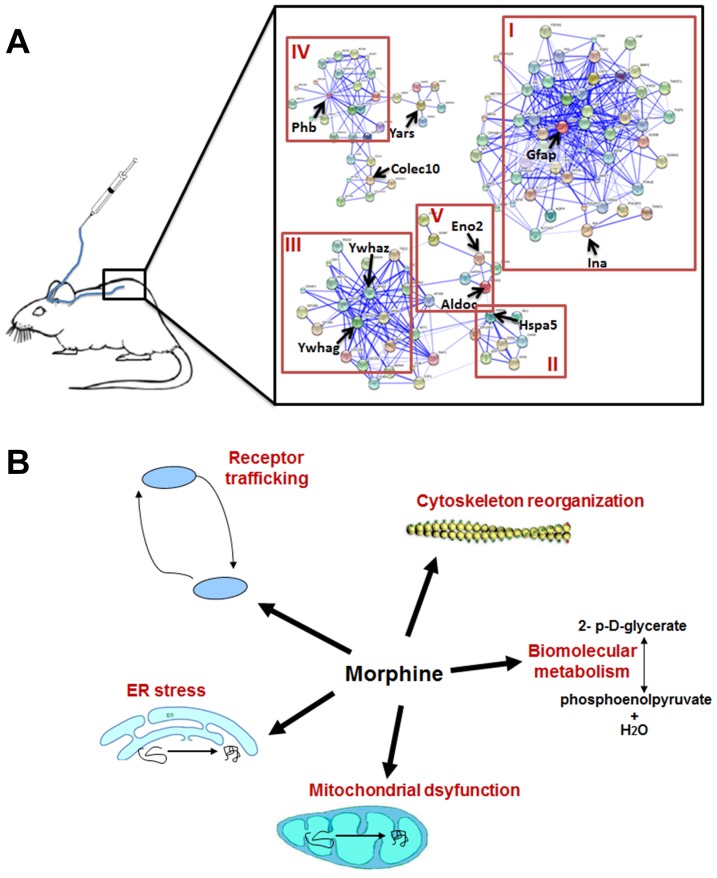
Male Sprague-Dawley rats (250–300 g) were used in this study. As shown in Fig. 1A, we first implanted a polyethylene-10 catheter into the subarachnoid space of the rats at the rostral level of the spinal cord lumbar enlargement segments as described previously [4], [5], [15]–[17]. The animals were allowed to recover for a week; animals that developed neurologic deficits postoperatively were removed from the study. After recovering from the catheter implantation, the rats were injected intrathecally through the catheter twice daily with saline (10 µL; control group; n = 6) or morphine sulfate (20 µg/10 µL saline, Sigma, St. Louis, MO; n = 6) for 4 consecutive days [5], [18]. On day 5, we injected rats in both groups with morphine sulfate (20 µg/10 µL) to evaluate the analgesic potency [5], [19].
Figure 1. Experimental protocol, nociception, and morphine efficacy.
(A) Flow chart of the experimental protocol. (B) Rats were administered twice daily injections of saline (control) or morphine for 4 days. The test morphine injection on day 5 significantly increased the paw withdrawal latency (PWL) of both hind paws in the control group, but not in the morphine-treated group. (C) The calculated maximal possible analgesic effect (MPAE) in both hind paws of morphine-treated rats was significantly lower than that in control rats. *p<0.05 vs. control group.
Nociception tests for morphine efficacy
Nociception tests were carried out at baseline (1 day before the first morphine or saline injection) and on day 5 (30 min after the final injection) as shown in Fig. 1A. Noxious radiant heat (Model 33B Analgesia Meter; IITC Life Science, Woodland Hills, CA, USA) was applied to the plantar surface of each hind paw of the animals. Paw withdrawal latency (PWL) of both hind paws was measured as the time between heat application and paw withdrawal. A cutoff time for the heat application was set to 20 s to prevent tissue damage to the paw [5].
Final and baseline PWLs were compared and used to calculate maximal possible analgesic effect (MPAE) of morphine. MPAE was calculated by the formula: [(final PWL – baseline PWL)/(cutoff time – baseline PWL)] x 100% [5].
Sample preparation and two-dimensional gel electrophoresis (2-DE)
After nociception testing, the animals were killed and lumbar enlargement segments of spinal cord were harvested. The spinal tissues were immediately lysed and homogenized with a sonication probe in 0.5 mL of lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS, 1% DTT, and 0.5% IPG buffer [pH 3–10]). The lysates were centrifuged at 15000 g for 15 min at room temperature to remove insoluble debris. Protein concentration in the supernatant was determined by a modified Bradford method [20].
The spinal proteins (250 µg) from control and morphine-treated groups were loaded onto IPG strips (Immobiline DryStrip 3–10, GE Healthcare, Piscataway, NJ, USA) for simultaneous rehydration. Isoelectric focusing was performed by using a voltage-time program of 50 V for 12 h, 500 V for 1 h, 1000 V for 1 h, and 7000 V to give a total of 140,000 V-h. Immediately after focusing, the IPG strips were equilibrated for 15 min in equilibration buffer (6 M urea, 2% sodium dodecyl sulfate [SDS]; 50 mM Tris [pH 8.4], and 30% glycerol) containing 1% dithiothreitol, and then for 15 min in equilibration buffer containing 2.5% iodoacetamide. The second dimension separation was carried out at 15°C with a vertical electrophoresis system (GE Healthcare) in 1 mm 12.5% acrylamide gels run at 20 mA/gel.
Gel staining and image analysis
After electrophoresis, the 2-DE gels were subjected to fluorescence staining with Pro-Q Diamond phosphoprotein dye (Invitrogen, Carlsbad, CA, USA) for detection of protein phosphorylation levels [21]. Briefly, the gels were fixed in fixation solution (50% methanol, 10% acetic acid), washed with distilled water twice, and then stained by the Pro-Q Diamond dye for 4 hours. The gels were destained in three successive washes of destaining solution (20% acetonitrile, 50 mM sodium acetate [pH 4]) and three washes of distilled water. Images were obtained by scanning the 2-DE gels with a Typhoon Trio laser scanner (GE Healthcare). For measuring total protein levels, the stained gels were washed with methanol solution (50% methanol, 10% acetic acid), to remove Pro-Q Diamond dye, and were restained with SYPRO Ruby fluorescent dye according to the manufacturer's protocol. Gel images were obtained again by scanning the 2-DE gels with a Typhoon Trio laser scanner.
Spot detection, gel matching, and spot quantification were carried out with 2-DE gel analysis software (ImageMaster 2D platinum, GE Healthcare). To correctly estimate phosphorylation levels, we normalized the phosphorylation intensities of an individual protein revealed by Pro-Q Diamond dye to the total expression level of the same protein revealed by SYPRO Ruby fluorescent dye [21]. The molecular weight (Mr) and isoelectric point (pI) of each protein spot were estimated by the software based on the positions of standard markers and standard pI positions, respectively.
In-gel digestion, matrix-assisted laser desorption/ionization-time-of-flight (MADLI-TOF) mass spectroscopy (MS) analysis, and protein identification
Proteins whose phosphorylation level differed significantly between control and morphine-treated groups were excised from the 2-DE gels and digested in the gel with trypsin. The digested proteins were subjected to MALDI-TOF MS analysis (Autoflex II, Bruker Daltonics, Bremen, Germany) to obtain the peptide mass fingerprint (PMF). Each mass spectrum was obtained from the average of signals generated from at least 500 laser shots. The PMFs were processed by using Flexanalysis™ and Biotools™ software (Bruker Daltonics) and were used to search the UniProt database (http://www.uniprot.org/) by using the MS-Fit on-line search engine (http://prospector.ucsf.edu/). For each PMF search to identify a protein, the mass tolerance was set at 150 ppm, and one missed tryptic cleavage was allowed.
Pull-down assays of phosphopeptide and phosphoprotein
To identify the types and sites of phosphorylation in the most significantly changed protein (i.e. 14-3-3 proteins), we performed pull-down assays at both peptide and protein levels. For pull-down assay of a phophopeptide, the selected protein was in-gel digested into many peptides, and phosphopeptides of the digested protein were precipitated by using Phos-trap™ magnetic beads (Perkin Elmer, San Jose, CA, USA) according to the manufacturer's protocol [22]. MALDI-TOF MS was then used to reveal the precipitated phosphopeptides as well as the original total peptides.
For pull-down assay of phophoproteins, we homogenized spinal cord with RIPA buffer (150 mM NaCl, 0.05% SDS, 1% Triton X-100, 1 mM sodium orthovanadate, 5 mM sodium fluoride, 1x Roche protease inhibitor cocktail, and 50 mM Tris-HCl [pH 7.5]) and precipitated phosphoproteins from the lysate by using a mixed mouse monoclonal anti-phospho-serine and anti-phospho-threonine antibody (Chemicon International, Temecula, CA, USA). Precipitated proteins were then subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Western blotting was carried out sequentially by electroblotting proteins onto polyvinylidene difluoride membranes, incubating the membranes with a mouse monoclonal anti-14-3-3 antibody (Chemicon International), and probing with anti-mouse HRP-conjugated secondary antibody. Bands were visualized with an ECL detection kit (Millipore, Billerica, MA, USA). The lysates also were subjected to SDS-PAGE and Western blotting to check the total amount of 14-3-3 protein and glyceraldehyde 3-phosphate dehydrogenase, which served as input controls.
Bioinformatics analysis
To further estimate the impact of morphine on biological networks, the bioinformatics tool STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) was used to elucidate biological networks regulated by the proteomics-identified phosphoproteins [23]–[25]. The networks were generated by algorithmically assembling the identified phosphoproteins and their interacting proteins from the STRING database. The biological networks were then classified into clusters by protein function [23]–[25].
Statistics
Student's t-test was used for the statistical comparison of data from control and morphine-treated groups. Differences were considered significant at p<0.05. The data are presented as the mean ± SD.
Results
Establishment of morphine tolerance in rats
PWLs and MPAEs were measured to ensure the development of morphine tolerance in rats after repeated injections of morphine. As shown in Fig. 1B, the baseline PWLs were similar between the control and morphine-treated rats (p>0.05), whereas the final PWLs on day 5 were significantly shortened in morphine-treated rats as compared to those in control rats (p<0.05). Because PWL inversely correlates with the extent of pain sensation, the shortened PWLs indicate a reduced analgesic effect of morphine. To ensure the development of morphine tolerance, we also quantified the degree of morphine antinociception by calculating MPAEs. As shown in Fig. 1C, MPAE was significantly less in the morphine-treated rats than in the control rats in both left and right paws (p<0.05). Again, this quantitative data confirmed that the analgesic effect of morphine was reduced after repeated morphine injections and verified morphine tolerance.
Phosphoproteome profiles of spinal cord from control and morphine-treated rats
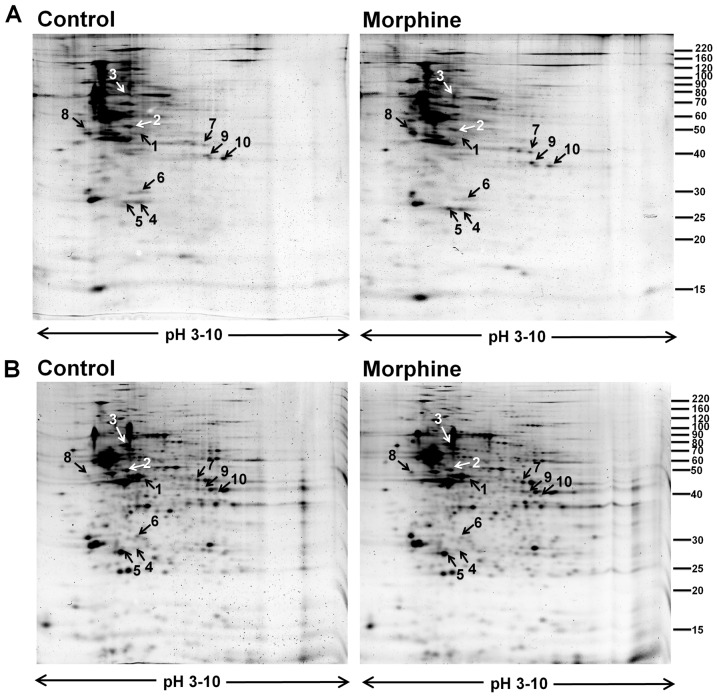
Comparative 2-DE-based proteomic analysis revealed a marked difference in phosphorylation pattern of spinal cord proteins between control and morphine-treated rats. Patterns of protein phosphorylation are shown by the representative 2-DE gels stained with ProQ Diamond phosphorylation detection kits (Fig. 2A), and patterns of total protein expression are shown by the same representative 2-DE gels restained with SyproRuby protein staining dye (Fig. 2B). Proteins that exhibited a significant difference in phosphorylation level between control (n = 6) and morphine-treated (n = 6) rats are indicated by arrows. Of those, four proteins were hypophosphorylated and six were hyperphosphorylated in the morphine-treated rats as compared to the control rats.
Figure 2. Representative 2-DE gel maps of phosphorylated proteins and total proteins from spinal cord of control and morphine-treated rats.
(A) Typical 2-DE gels representing phosphorylated proteins from control (left) and morphine-treated (right) rats; gels were stained with a ProQ Diamond phosphorylation detection dye. (B) The same pair of 2-DE gels from panel A restained with SyproRuby fluorescent dye to show the total protein expression profiles. The protein phosphorylation intensities of the upper gels in panel A were normalized to total protein expression intensities obtained from the lower gels in panel B. The proteins that showed a significant difference in the normalized phosphorylation level are indicated by arrows and labeled with the same numbers used in Table 1. The molecular mass is indicated on the right, and the pI range is shown at the bottom of each gel.
Quantification and identification of differentially phosphorylated proteins
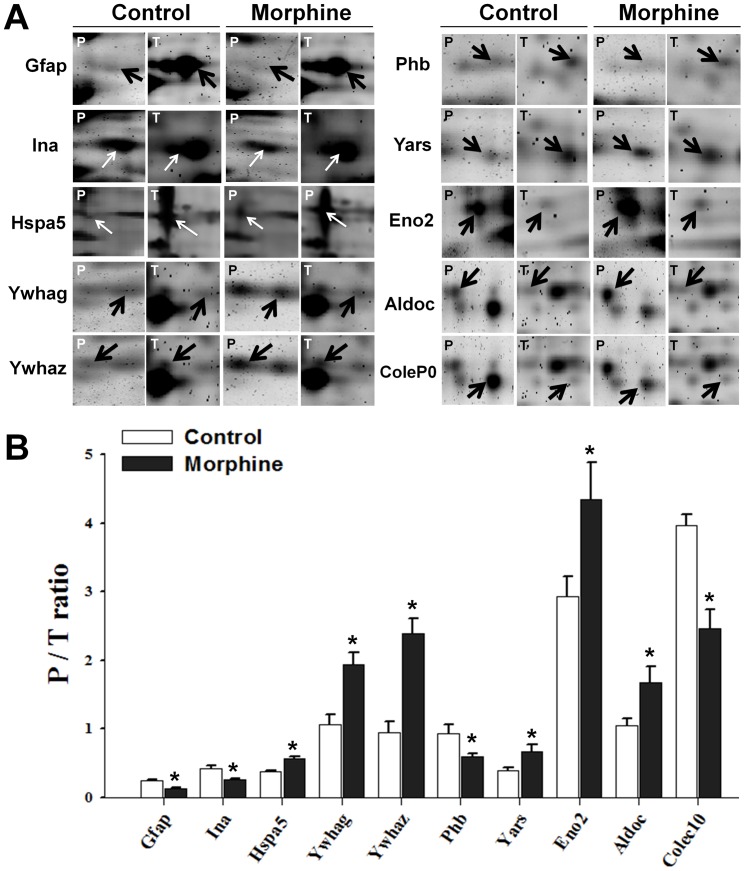
The magnified images in Fig. 3A show phosphorylation level (P) and total amount (T) of individual protein spots in control and morphine-treated groups; intensities of protein phosphorylation were divided by corresponding intensities of total protein to give the normalized phosphorylation levels (P/T ratios) shown in Fig. 3B [21]. Differentially phosphorylated proteins were identified by PMF with MALDI-TOF MS, and their various characteristics are shown in Table 1. The proteins included glial fibrillary acidic protein (Gfap), alpha-internexin (Ina), heat shock 70 kDa protein 5 (Hspa5), 14-3-3 protein gamma (Ywhag), 14-3-3 protein zeta/delta (Ywhaz), prohibitin (Phb), tyrosyl-tRNAsynthetase (Yars), gamma-enolase (Eno2), fructose-bisphosphatealdolase C (Aldoc) and collectin sub-family member 10 (Colec10). Phosphorylation levels of Gfap, Ina, Phb, and Colec10 were decreased in the morphine-treated group, whereas phosphorylation levels of the other six proteins were increased.
Figure 3. Quantification of differentially phosphorylated proteins.
(A) Magnified images of protein spots showing phosphorylation (P) and total amount (T) of the 10 differentially phosphorylated proteins in control and morphine-treated rats. The differentially phosphorylated proteins are indicated by arrows in each magnified image. (B) Statistical and quantification data (P/T ratios) of individual proteins. All differences between the control and morphine-treated groups are significant at the p<0.05 level.
Table 1. Identified proteins.
| Spot No. | MOWSE score | Accession No. | Protein name abbreviation | Protein name | Theoretical Mr (kDa)/pI | Sequence coverage (%) |
| 1 | 16300000 | P47819 | Gfap | Glial fibrillary acidic protein | 49.957/5.4 | 58.8 |
| 2 | 312000000 | P23565 | Ina | Alpha-internexin | 56.116/5.2 | 58.8 |
| 3 | 5574 | P06761 | Hspa5 | Heat shock 70 kDa protein 5 | 72.348/5.1 | 43.1 |
| 4 | 9645 | P61983 | Ywhag | 14-3-3 protein gamma | 28.303/4.8 | 41.7 |
| 5 | 139899 | P63102 | Ywhaz | 14-3-3 protein zeta/delta | 27.771/4.7 | 51.4 |
| 6 | 39333 | P67779 | Phb | Prohibitin | 29.82/5.6 | 37.5 |
| 7 | 204 | Q4KM49 | Yars | Tyrosyl-tRNA synthetase | 59.116/6.6 | 20.1 |
| 8 | 16777 | P07323 | Eno2 | Gamma-enolase | 47.141/5.0 | 27.9 |
| 9 | 1253 | P09117 | Aldoc | Fructose-bisphosphate aldolase C | 39.284/6.7 | 40.5 |
| 10 | 45.3 | D4A7F6 | Colec10 | Collectin sub-family member 10 | 30.628/7.5 | 11.2 |
The spot number refers to the numbers in Fig. 2. The MOWSE (MOlecular Weight SEarch) score is used to identify proteins from the molecular weight of peptides produced by proteolytic digestion. Mr, theoretical molecular mass; pI, isoelectric point. The sequence coverage of matching peptides was calculated by using Biotools™ software.
Identification of phosphoproteins involved in neuroplasticity
Gfap (Fig. 2, spot 1; Table 1), a glia cell-specific cytoskeleton protein [26], and Ina (Fig. 2, spot 2, Table 1), a neuron-specific cytoskeleton protein [27], were hypophosphorylated after morphine treatment. Both cytoskeletal proteins play roles in neuroplasticity.
Identification of chaperones
Chaperones are involved in maintaining proper conformations of and modulating activities of other proteins. After repeated injection of morphine, phosphorylation levels of one endoplasmic reticulum (ER)-specific chaperone (Hspa5; Fig. 2, spot 3; Table 1) [28] and two 14-3-3 proteins (Fig. 2, spots 4 and 5; Table 1) [29] were increased.
Identification of signaling scaffold protein
Scaffold proteins are key regulators of many signaling pathways. Repeated treatment with morphine reduced the phosphorylation level of Phb (Fig. 2, spot 6; Table 1), a scaffold protein that controls the signal transduction of PI3K/Akt, TGF-beta, and Ras/MAPK/ERK [30].
Identification of enzymes involved in biomolecular metabolism
The phosphorylation levels of three metabolic enzymes were altered, including two glycolysis enzymes, Eno2 (Fig. 2, spot 8; Table 1) and Aldoc (Fig. 2, spot 9; Table 1) [31], and one protein synthesis-related enzyme Yars (Fig. 2, spot 7; Table 1).
Identification of types and sites of phosphorylation using pull-down assays
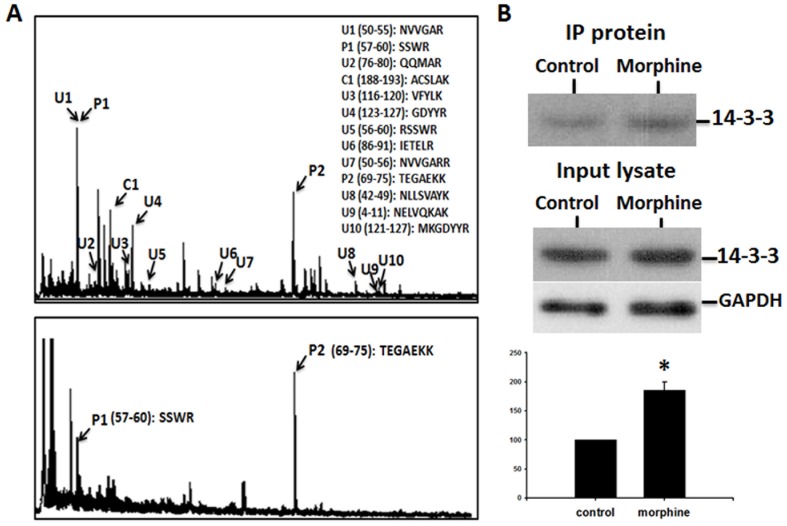
Pull-down assays were performed to identify the types and sites of phosphorylation in the most significantly changed protein (i.e. 14-3-3 proteins) in 2-DE. Phosphopeptide-enriching magnetic beads induced the precipitation of two major phosphopeptides (P1 and P2 in Fig. 4) from the total peptides of the in-gel digested 14-3-3 protein Ywhaz (spot 5). Based on their amino acid sequences, these peptides were predicted to be serine- and threonine-phosphorylated (Fig. 4A).
Figure 4. Phosphorylation of 14-3-3 proteins as shown by pull-down assays of phosphopeptide and phosphoprotein.
(A) MALDI-TOF MS spectrum of total peptides derived from trypsin digestion of 14-3-3 protein Ywhaz (top panel), and a spectrum of phophopeptides precipitated from total peptides of the digested 14-3-3 protein (bottom panel). The phosphorylated residues represented by the peaks were predicted to be serine and threonine. (B) Upper panel: Western blot showing that more 14-3-3 protein was precipitated by anti-phospho-serine/anti-phospho-threonine antibodies in morphine-treated spinal cord than in control spinal cord. Middle panel: Western blots showing that total 14-3-3 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) proteins in the lysates of spinal cord were similar in control and morphine-treated rats. Lower panel: Statistical and quantification data of phosphorylation of 14-3-3 proteins.
Immunoprecipitation of the phosphorylated proteins from total lysates of spinal cord samples and detection by Western blot analysis revealed that 14-3-3 proteins were more abundant in spinal cords from morphine-treated rats than in those from control rats, and confirmed the prediction that 14-3-3 proteins were serine- and threonine-phosphorylated (Fig. 4B).
Biological networks regulated by the morphine-affected phosphoproteins
Bioinformatics analysis was used to estimate the biological networks impacted by the 10 proteomics-identified phosphoproteins. The biological network clusters, shown in Fig. 5, consisted of cytoskeleton reorganization and neuroplasticity (cluster I), protein folding (cluster II), protein modulation (cluster III), signal transduction (cluster IV), and biomolecular metabolism (cluster V).
Figure 5. Schematic diagrams of protein-interaction networks and morphine effects.
(A) Schematic diagram showing protein-interaction networks affected by the morphine-regulated phosphoproteins. The networks were mapped by using the on-line bioinformatics analysis tool STRING (http://string.embl.de/) and can be classified into five different functional clusters: (I) cytoskeletal reorganization and neuroplasticity, (II) protein folding, (III) protein modulation, (IV) signal transduction, and (V) biomolecular metabolism. (B) Schematic summary showing morphine effects, including receptor trafficking, ER stress, mitochondrial dysfunction, biomolecular metabolism and cytoskeleton reorganization.
Discussion
To date, phosphoproteomics has not been used to analyze phosphorylation of spinal cord proteins after development of morphine tolerance. Our proteomics and bioinformatics data revealed that repeated intrathecal morphine injections dysregulated the phosphorylation of 10 proteins in rat spinal cord, impacting biological networks associated with various physiologic functions of the CNS. Our results are novel in that the identified phosphoproteins have not been reported previously to be associated with morphine tolerance.
The nervous system requires a degree of plasticity to respond to external stimuli and insults. This plasticity is achieved through cytoskeletal reorganization, which is controlled by phosphorylation of cytoskeletal proteins [32]–[34]. Our study showed that phosphorylation states of two cytoskeletal proteins in the spinal cord, i.e. Gfap and Ina, were altered by repeated administration of morphine. GFAP is an Intermediate filament protein specifically expressed in astrocytes in the CNS, and it serves as a sensitive and specific indicator of CNS plasticity in neurotoxic conditions [35], [36]. Ina is another intermediate filament protein expressed only in neurons, and can cause CNS plasticity by facilitating axonal neurite elongation [37]. These data are consistent with previous reports showing that morphine administration impact GFAP expression in certain brain areas [38], and support previously published evidence showing that morphine tolerance is actually a disorder of neuroplasticity [39], [40].
We also identified several chaperone proteins in this study. Chaperones play a key role in preventing their target proteins from misfolding and aggregating into nonfunctional structures; hence, they ensure proper activity of the target proteins [41]. The phosphorylation level of an ER-specific chaperone, Hspa5 (also called GRP78), was significantly altered in the spinal cord after development of morphine tolerance [42], [43]. Hspa5 is a major ER chaperone controlling the protein quality in the ER and protecting cells from ER stress, which is characterized by accumulation of misfolded proteins in ER [42], [43]. Various stresses can upregulate the expression of Hspa5, and the overexpressed Hspa5 within and outside the ER to play a critical role in cell viability [44]–[46]. For example, Hspa5 protects neurons and astrocytes against mitochondria dysfunction and stress-induced apoptosis [44], [46]. The effect of morphine on Hspa5 is consistent with previous reports demonstrating that another ER chaperone, BiP, also plays a pathophysiologic role in the development of morphine tolerance [47].
Two of the identified chaperones belong to the 14-3-3 protein family [48], [49]. 14-3-3 proteins are able to bind to the phosphorylated motifs of their partner proteins to affect protein activities [29]. Through functional modulation of the binding partners, 14-3-3 proteins are involved in multiple processes running in the cell, including metabolism, apoptosis, cell cycle and gene transcription [50], [51]. It has been known that posttranslational modifications playing important roles in regulation of 14-3-3 activity, and this regulation plays an important role in cytoskeleton reorganization of intermediate filaments in neurons [52]. Especially, 14-3-3 proteins modulate the sensitivity and trafficking of opioid and NMDA receptors [53], [54], both of which are known to be involved in the development of morphine tolerance [39]. Thus, the dysregulated phosphorylation of 14-3-3 proteins seen in this study might contribute to the pathophysiology of morphine tolerance through opioid and/or NMDA receptors.
Like chaperones, scaffold proteins are a type of regulatory protein that modulates cellular processes through interaction with multiple partner proteins. In our study, we identified Phb, a scaffold protein originally found in mitochondria [55]. Within mitochondria, Phb is able to maintain mitochondrial integrity and suppress free radical production [56]. However, Phb also has functions outside of mitochondria, such as controlling proliferation, apoptosis, and transcription [57]. In addition, Phb is neuroprotective against stress-induced neuronal death [56], and plays a role in modulating PI3K/Akt and Ras/MAPK/ERK signal transduction [30]. The altered phosphorylation state of Phb in morphine-treated rats suggests that morphine influences some of the known functions of Phb in the spinal cord.
In addition to the structural and regulatory proteins described above, our results also demonstrated that local injection of morphine into spinal cord disturbed the phosphorylation of two glycolysis metabolic enzymes (Eno2 and Aldoc) in the injected spinal area. This finding is consistent with previous reports showing that systematic treatment of morphine dysregulates metabolic enzymes and alters the functional state of carbohydrate metabolism in both the CNS and peripheral organs [58]–[60]. Interestingly, Eno2 is an enzyme highly expressed in CNS, and has neurotrophic and neuroprotective effects on a broad spectrum of CNS neurons [61], [62]. It can be inferred that the neuronal effects may also be associated with the development of morphine tolerance.
In conclusion, our proteomics data showed that repeated intrathecal injection of morphine dysregulated the phosphorylation of 10 proteins in the spinal cord. Bioinformatics analysis revealed five functional networks of proteins that are affected and known to be involved in cytoskeletal reorganization, neuroplasticity, protein folding and modulation, signal transduction, and biomolecular metabolism (Fig. 5A). These proteins are known to be expressed in different subcellular organells, associating with neuroplasticity, ER stress, mitochondrial dsyfunction, receptor trafficking and neuroprotection (Fig. 5B). Our data may shed light on the multiple phosphoproteome mechanisms that underlie the development of morphine tolerance.
Funding Statement
This work was supported by grants NSC 99-2628-B-016 -013 -MY3 from the National Science Council and TSGH-C97-13-S03, TSGH-C98-05-S03, and TSGH-C98-05-S03 from the Tri-Service General Hospital, Taiwan, R.O.C. to W.-J.L. and by the NIH grants RO1 NS058886 and NS072206, the Rita Allen Foundation, Mr. David Koch and the Patrick C. Walsh Prostate Cancer Research Fund, and the Blaustein Pain Research Fund to Y.X.T. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, et al. (2001) Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol 19: 2542–2554. [DOI] [PubMed] [Google Scholar]
- 2. Gerber HR (2003) Intrathecal morphine for chronic benign pain. Best Pract Res Clin Anaesthesiol 17: 429–442. [DOI] [PubMed] [Google Scholar]
- 3. Osenbach RK, Harvey S (2001) Neuraxial infusion in patients with chronic intractable cancer and noncancer pain. Curr Pain Headache Rep 5: 241–249. [DOI] [PubMed] [Google Scholar]
- 4. Shui HA, Ho ST, Wang JJ, Wu CC, Lin CH, et al. (2007) Proteomic analysis of spinal protein expression in rats exposed to repeated intrathecal morphine injection. Proteomics 7: 796–803. [DOI] [PubMed] [Google Scholar]
- 5. Liaw WJ, Zhang B, Tao F, Yaster M, Johns RA, et al. (2004) Knockdown of spinal cord postsynaptic density protein-95 prevents the development of morphine tolerance in rats. Neuroscience 123: 11–15. [DOI] [PubMed] [Google Scholar]
- 6. Gutstein HB, Trujillo KA (1993) MK-801 inhibits the development of morphine tolerance at spinal sites. Brain Res 626: 332–334. [DOI] [PubMed] [Google Scholar]
- 7. Menard DP, van Rossum D, Kar S, Jolicoeur FB, Jhamandas K, et al. (1995) Tolerance to the antinociceptive properties of morphine in the rat spinal cord: alteration of calcitonin gene-related peptide-like immunostaining and receptor binding sites. J Pharmacol Exp Ther 273: 887–894. [PubMed] [Google Scholar]
- 8. Hauser KF, Mangoura D (1998) Diversity of the endogenous opioid system in development. Novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol 5: 437–449. [PubMed] [Google Scholar]
- 9. Berhow MT, Hiroi N, Nestler EJ (1996) Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci 16: 4707–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olasmaa M, Terenius L (1988) Prolonged exposure of human neuroblastoma SH-SY5Y cell line to morphine and oxotremorine modulates signal transduction in adenylate cyclase system. Brain Res 475: 291–296. [DOI] [PubMed] [Google Scholar]
- 11. Kim SY, Chudapongse N, Lee SM, Levin MC, Oh JT, et al. (2004) Proteomic analysis of phosphotyrosyl proteins in the rat brain: effect of butorphanol dependence. J Neurosci Res 77: 867–877. [DOI] [PubMed] [Google Scholar]
- 12. Kim SY, Chudapongse N, Lee SM, Levin MC, Oh JT, et al. (2005) Proteomic analysis of phosphotyrosyl proteins in morphine-dependent rat brains. Brain Res Mol Brain Res 133: 58–70. [DOI] [PubMed] [Google Scholar]
- 13. Mumby M, Brekken D (2005) Phosphoproteomics: new insights into cellular signaling. Genome Biol 6: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ram PT, Mendelsohn J, Mills GB (2012) Bioinformatics and systems biology. Mol Oncol 6: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yaksh TL, Rudy TA (1976) Analgesia mediated by a direct spinal action of narcotics. Science 192: 1357–1358. [DOI] [PubMed] [Google Scholar]
- 16. Tao YX, Hassan A, Haddad E, Johns RA (2000) Expression and action of cyclic GMP-dependent protein kinase Ialpha in inflammatory hyperalgesia in rat spinal cord. Neuroscience 95: 525–533. [DOI] [PubMed] [Google Scholar]
- 17. Tao F, Tao YX, Gonzalez JA, Fang M, Mao P, et al. (2001) Knockdown of PSD-95/SAP90 delays the development of neuropathic pain in rats. Neuroreport 12: 3251–3255. [DOI] [PubMed] [Google Scholar]
- 18. Mao J, Price DD, Mayer DJ (1994) Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci 14: 2301–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tao YX, Johns RA (2002) Activation and up-regulation of spinal cord nitric oxide receptor, soluble guanylate cyclase, after formalin injection into the rat hind paw. Neuroscience 112: 439–446. [DOI] [PubMed] [Google Scholar]
- 20.Ramagli L (1999) Quantifying protein in 2-D PAGE solubilization buffer. In: Link AJ, editor. 2-D Proteome analysis protocols. Totowa, NJ: Humana Press. pp. 99–103.
- 21. Chan CC, Shui HA, Wu CH, Wang CY, Sun GH, et al. (2009) Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J Proteome Res 8: 5382–5386. [DOI] [PubMed] [Google Scholar]
- 22. Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJ (2005) Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics 4: 873–886. [DOI] [PubMed] [Google Scholar]
- 23. Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, et al. (2009) STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res 37: D412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shui HA, Hsia CW, Chen HM, Chang TC, Wang CY (2012) Proteomics and bioinformatics analysis of lovastatin-induced differentiation in ARO cells. J Proteomics 75: 1170–1180. [DOI] [PubMed] [Google Scholar]
- 25. Chen PH, Wang CY, Hsia CW, Ho MY, Chen A, et al. (2011) Impact of taxol on dermal papilla cells—a proteomics and bioinformatics analysis. J Proteomics 74: 2760–2773. [DOI] [PubMed] [Google Scholar]
- 26. Rodnight R, Goncalves CA, Wofchuk ST, Leal R (1997) Control of the phosphorylation of the astrocyte marker glial fibrillary acidic protein (GFAP) in the immature rat hippocampus by glutamate and calcium ions: possible key factor in astrocytic plasticity. Braz J Med Biol Res 30: 325–338. [DOI] [PubMed] [Google Scholar]
- 27. Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, et al. (2006) Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci 26: 10006–10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guzel E, Basar M, Ocak N, Arici A, Kayisli UA (2011) Bidirectional interaction between unfolded-protein-response key protein HSPA5 and estrogen signaling in human endometrium. Biol Reprod 85: 121–127. [DOI] [PubMed] [Google Scholar]
- 29. Yaffe MB (2002) How do 14-3-3 proteins work? — Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett 513: 53–57. [DOI] [PubMed] [Google Scholar]
- 30. Mishra S, Ande SR, Nyomba BL (2010) The role of prohibitin in cell signaling. FEBS J 277: 3937–3946. [DOI] [PubMed] [Google Scholar]
- 31. Hafner A, Obermajer N, Kos J (2012) gamma-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem J 443: 439–450. [DOI] [PubMed] [Google Scholar]
- 32. Rex CS, Gavin CF, Rubio MD, Kramar EA, Chen LY, et al. (2010) Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation. Neuron 67: 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sekino Y, Kojima N, Shirao T (2007) Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int 51: 92–104. [DOI] [PubMed] [Google Scholar]
- 34. Ferrer-Alcon M, Garcia-Sevilla JA, Jaquet PE, La Harpe R, Riederer BM, et al. (2000) Regulation of nonphosphorylated and phosphorylated forms of neurofilament proteins in the prefrontal cortex of human opioid addicts. J Neurosci Res 61: 338–349. [DOI] [PubMed] [Google Scholar]
- 35. O'Callaghan JP, Sriram K (2005) Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf 4: 433–442. [DOI] [PubMed] [Google Scholar]
- 36. Lewis GP, Fisher SK (2003) Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol 230: 263–290. [DOI] [PubMed] [Google Scholar]
- 37. Shea TB, Beermann ML (1999) Neuronal intermediate filament protein alpha-internexin facilitates axonal neurite elongation in neuroblastoma cells. Cell Motil Cytoskeleton 43: 322–333. [DOI] [PubMed] [Google Scholar]
- 38. Alonso E, Garrido E, Diez-Fernandez C, Perez-Garcia C, Herradon G, et al. (2007) Yohimbine prevents morphine-induced changes of glial fibrillary acidic protein in brainstem and alpha2-adrenoceptor gene expression in hippocampus. Neurosci Lett 412: 163–167. [DOI] [PubMed] [Google Scholar]
- 39. Ueda H, Ueda M (2009) Mechanisms underlying morphine analgesic tolerance and dependence. Front Biosci 14: 5260–5272. [DOI] [PubMed] [Google Scholar]
- 40. Liaw WJ, Zhu XG, Yaster M, Johns RA, Gauda EB, et al. (2008) Distinct expression of synaptic NR2A and NR2B in the central nervous system and impaired morphine tolerance and physical dependence in mice deficient in postsynaptic density-93 protein. Mol Pain 4: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaudhuri TK, Paul S (2006) Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J 273: 1331–1349. [DOI] [PubMed] [Google Scholar]
- 42. Yamaguchi Y, Larkin D, Lara-Lemus R, Ramos-Castaneda J, Liu M, et al. (2008) Endoplasmic reticulum (ER) chaperone regulation and survival of cells compensating for deficiency in the ER stress response kinase, PERK. J Biol Chem 283: 17020–17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quinones QJ, de Ridder GG, Pizzo SV (2008) GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol 23: 1409–1416. [DOI] [PubMed] [Google Scholar]
- 44. Ouyang YB, Xu LJ, Emery JF, Lee AS, Giffard RG (2011) Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion 11: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim S, Wang M, Lee AS, Thompson RF (2011) Impaired eyeblink conditioning in 78 kDa-glucose regulated protein (GRP78)/immunoglobulin binding protein (BiP) conditional knockout mice. Behav Neurosci 125: 404–411. [DOI] [PubMed] [Google Scholar]
- 46. Wang M, Ye R, Barron E, Baumeister P, Mao C, et al. (2010) Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ 17: 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dobashi T, Tanabe S, Jin H, Mimura N, Yamamoto T, et al. (2010) BiP, an endoplasmic reticulum chaperone, modulates the development of morphine antinociceptive tolerance. J Cell Mol Med 14: 2816–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Exley R, Moroni M, Sasdelli F, Houlihan LM, Lukas RJ, et al. (2006) Chaperone protein 14-3-3 and protein kinase A increase the relative abundance of low agonist sensitivity human alpha 4 beta 2 nicotinic acetylcholine receptors in Xenopus oocytes. J Neurochem 98: 876–885. [DOI] [PubMed] [Google Scholar]
- 49. Foucault I, Liu YC, Bernard A, Deckert M (2003) The chaperone protein 14-3-3 interacts with 3BP2/SH3BP2 and regulates its adapter function. J Biol Chem 278: 7146–7153. [DOI] [PubMed] [Google Scholar]
- 50. Obsilova V, Silhan J, Boura E, Teisinger J, Obsil T (2008) 14-3-3 proteins: a family of versatile molecular regulators. Physiol Res 57 Suppl 3S11–21. [DOI] [PubMed] [Google Scholar]
- 51. Hermeking H, Benzinger A (2006) 14-3-3 proteins in cell cycle regulation. Semin Cancer Biol 16: 183–192. [DOI] [PubMed] [Google Scholar]
- 52. Sluchanko NN, Gusev NB (2010) 14-3-3 proteins and regulation of cytoskeleton. Biochemistry (Mosc) 75: 1528–1546. [DOI] [PubMed] [Google Scholar]
- 53. Chen BS, Roche KW (2009) Growth factor-dependent trafficking of cerebellar NMDA receptors via protein kinase B/Akt phosphorylation of NR2C. Neuron 62: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li JG, Chen C, Huang P, Wang Y, Liu-Chen LY (2012) 14-3-3 Zeta protein regulates anterograde transport of the human Kappa opioid receptor (hKOPR). J Biol Chem. [DOI] [PMC free article] [PubMed]
- 55. Merkwirth C, Langer T (2009) Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta 1793: 27–32. [DOI] [PubMed] [Google Scholar]
- 56. Zhou P, Qian L, D'Aurelio M, Cho S, Wang G, et al. (2012) Prohibitin reduces mitochondrial free radical production and protects brain cells from different injury modalities. J Neurosci 32: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Theiss AL, Sitaraman SV (2011) The role and therapeutic potential of prohibitin in disease. Biochim Biophys Acta 1813: 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sharma SK, Yashpal K, Fundytus ME, Sauriol F, Henry JL, et al. (2003) Alterations in brain metabolism induced by chronic morphine treatment: NMR studies in rat CNS. Neurochem Res 28: 1369–1373. [DOI] [PubMed] [Google Scholar]
- 59. Lelevich SV (2007) [Mechanisms of regulation of glycolytic enzymes in rat liver under morphine intoxication]. Biomed Khim 53: 284–289. [PubMed] [Google Scholar]
- 60. Lelevich SV (2004) [Dose-dependent effects of morphine hydrochloride on glycolysis in the rat liver]. Biomed Khim 50: 273–276. [PubMed] [Google Scholar]
- 61. Hafner A, Glavan G, Obermajer N, Zivin M, Schliebs R, et al. (2013) Neuroprotective role of gamma-enolase in microglia in a mouse model of Alzheimer's disease is regulated by cathepsin X. Aging Cell 12: 604–614. [DOI] [PubMed] [Google Scholar]
- 62. Hafner A, Obermajer N, Kos J (2010) gamma-1-syntrophin mediates trafficking of gamma-enolase towards the plasma membrane and enhances its neurotrophic activity. Neurosignals 18: 246–258. [DOI] [PubMed] [Google Scholar]