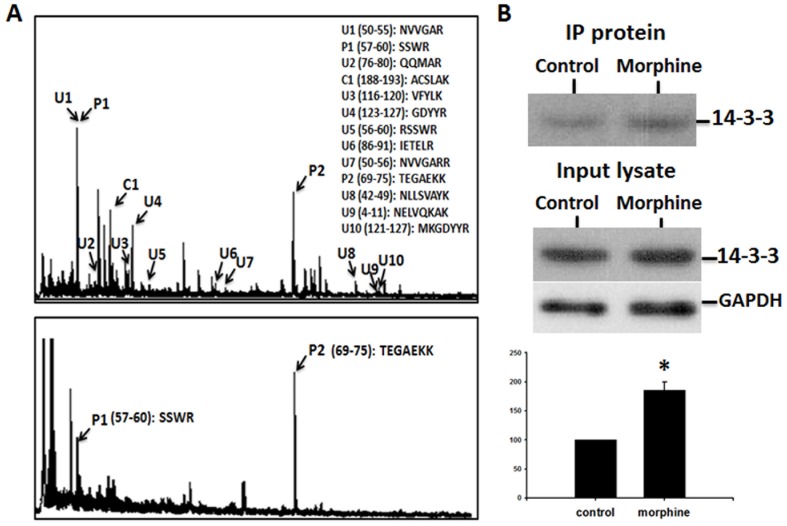
Figure 4. Phosphorylation of 14-3-3 proteins as shown by pull-down assays of phosphopeptide and phosphoprotein.
(A) MALDI-TOF MS spectrum of total peptides derived from trypsin digestion of 14-3-3 protein Ywhaz (top panel), and a spectrum of phophopeptides precipitated from total peptides of the digested 14-3-3 protein (bottom panel). The phosphorylated residues represented by the peaks were predicted to be serine and threonine. (B) Upper panel: Western blot showing that more 14-3-3 protein was precipitated by anti-phospho-serine/anti-phospho-threonine antibodies in morphine-treated spinal cord than in control spinal cord. Middle panel: Western blots showing that total 14-3-3 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) proteins in the lysates of spinal cord were similar in control and morphine-treated rats. Lower panel: Statistical and quantification data of phosphorylation of 14-3-3 proteins.