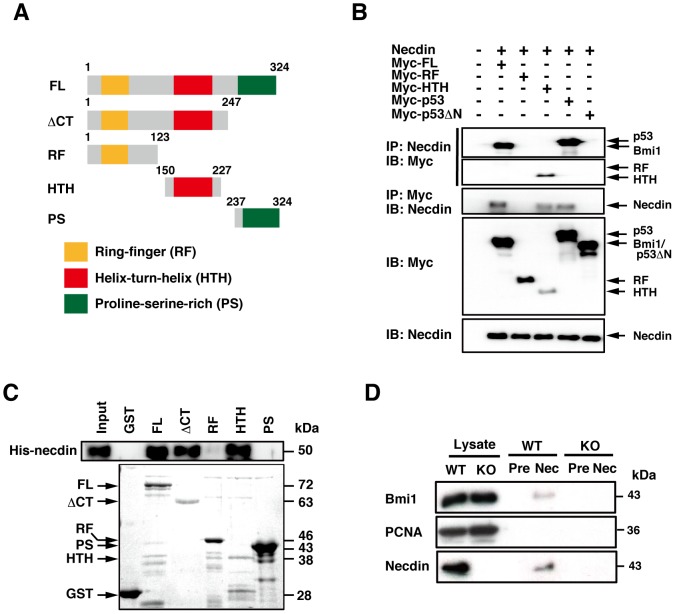
Figure 4. Necdin interacts with Bmi1 in vivo and in vitro.
(A) Bmi1 deletion mutants. Bmi1 full-length (FL), C-terminal deletion (ΔCT), and mutants containing the Ring finger (RF), helix-turn-helix (HTH) and proline/serine-rich (PS) domains are schematically shown. (B) Co-immunoprecipitation assay. HEK293A cells were transfected with expression vectors for necdin and Myc-tagged FL, RF, HTH, p53 (positive control), and p53ΔN (negative control). Cell lysates were immunoprecipitated (IP) and immunoblotted (IB) with anti-Myc (Myc) and anti-necdin (Necdin) antibodies. (C) In vitro binding assay. GST-Bmi1 mutants immobilized on glutathione-agarose were incubated with His-tagged necdin (His-necdin), and bound His-necdin was detected by immunoblotting with anti-necdin antibody (upper panel). GST-Bmi1 deletion mutants were stained with Coomassie Brilliant Blue (lower panel). Arrows indicate the predicted protein positions (B, C). (D) Co-immunoprecipitation assay for endogenous complex containing necdin and Bmi1 in primary NPCs. Lysates of NPCs prepared from E14.5 wild-type (WT) and necdin-null (KO) mice were immunoprecipitated with anti-necdin IgG (Nec) or control preimmune IgG (Pre). Bmi1, PCNA (negative control), and necdin were detected by Western blotting. Lysate, tissue lysate (10 µg).