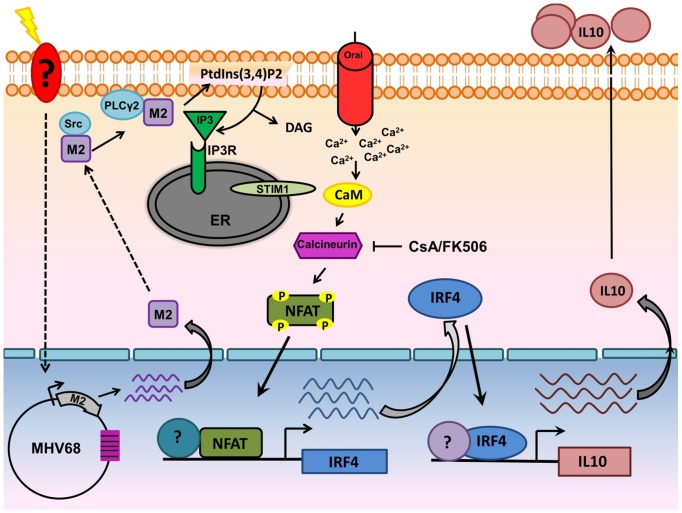
Figure 10. Model of M2 mediated IL-10.
Upon BCR engagement, multiple protein kinases including Src family kinases (Lyn,Fyn,Src,Blk,Yes), Syk, Btk get activated leading to the activation of PLCγ2.Activated PLCγ2 catalyzes the hydrolysis of phosphatidylinositol-4,5-biphosphate [(PtdIns(4,5)P2] to DAG and IP3. Binding of IP3 to its receptor on the ER membrane leads to release of ER Ca2+ stores. However, this increase in Ca2+ is only transient and following depletion of ER Ca2+ stores, an influx of extracellular Ca2+ occurs (termed as SOCE-Store Operated Calcium Entry). This prolonged and sustained increase in Ca2+ leads to activation of the Calcineurin-NFAT pathway. In the case of M2, Miranda et al have shown that M2 can interact with Fyn and PLCγ2. Our model suggests that interaction of M2 with proximal membrane signaling molecules such as Src kinase and/or PLCγ2 potentially mimics events that occur upon BCR engagement resulting in the Ca2+ mediated activation of the NFAT pathway. Induction of IRF4 by M2 is dependent, at least partially by the activation of NFAT pathway. IRF4 can activate regulatory elements in the IL-10 promoter locus (termed CNS-Conserved Noncoding Sequences) in T cells and in our case, in B cells as well. These series of events leads to eventual increase in the levels of IL-10 observed upon induction of M2.