FIGURE 3.
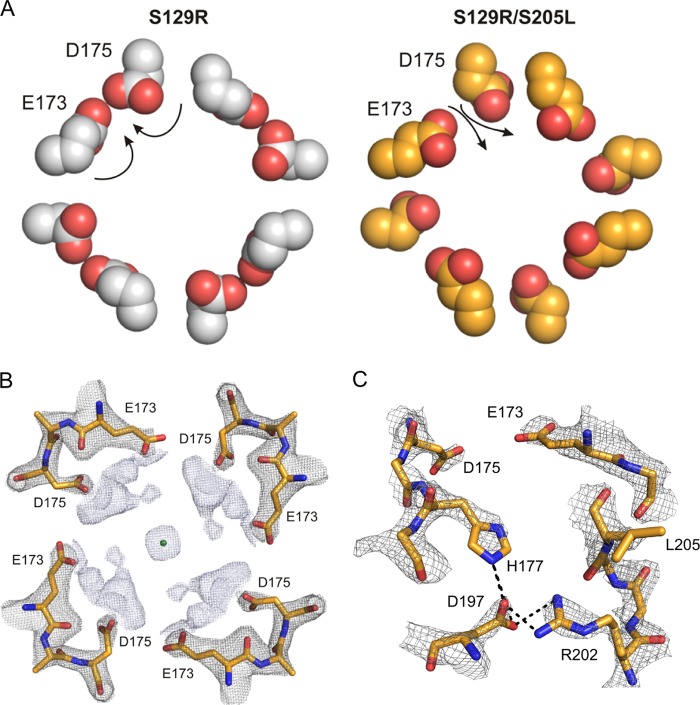
Mutation S205L results in a ring of negative charges within the cytoplasmic pore. A, top-down view of the arrangement of Glu173 and Asp175 relative to the cytoplasmic pore in the S129R (left, gray) and S129R/S205L (right, gold) mutants. Because of their interaction with His177, in the S129R structure, these residues are located within the intersubunit space. However, in the S205L mutant structure, they both point into the cytoplasmic pore. B, the cross-section of the 2Fo − Fc map shows a potassium ion (green sphere) coordinated by Glu173 and Asp175. The 2Fo − Fc map is contoured at σ2 for Glu173–Glu175 (gray mesh) and at σ1 for the potassium ion (light blue mesh). The potassium ion is probably hydrated, but no water molecules have been modeled around this ion. C, side view of the electron density at the cytoplasmic intersubunit interface in the S129R/S205L mutant channel in which His177 interacts with Asp197, thereby releasing Glu173 and Asp175 into the inner cavity. The 2Fo − Fc map is contoured at σ2.