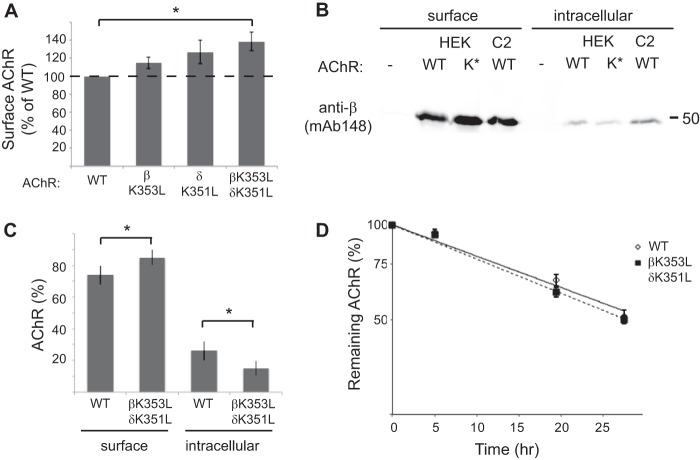
FIGURE 7.
Mutations in the retention motif increase surface trafficking of assembled AChR. A, HEK cells were transfected with wild-type AChR or receptor with single or combined βK353L and δK351L mutations, and surface levels of receptor were measured by 125I-labeled α-BuTx binding. Receptor with combined βK353 and δK351 mutations was expressed on the cell surface at significantly higher levels than wild-type AChR (138% of WT levels, p = 0.02, t test, n = 4). Smaller increases were observed with individual mutations of the β or δ subunits. Error bars, S.E. B, surface and intracellular AChR were isolated sequentially from HEK cells transfected with wild-type (WT) or βK353L/δK351L (K*)-AChR and from control C2 muscle cells. The isolates were then immunoblotted with anti-β subunit antibody (mAb148) to compare receptor levels. Compared with WT-AChR, more βK353L/δK351L-AChR was present in the surface pool and less in the intracellular pool. C, quantification of the immunoblotting experiments shows the relative percentages of surface and intracellular AChR. A significantly higher percentage of total βK353L/δK351L-AChR was expressed on the cell surface compared with WT-AChR (p = 0.02; t test, n = 4), consistent with decreased Golgi retention. D, wild-type and βK353L/δK351L-AChR were surface-labeled with 125I-α-BuTx, and receptor degradation was measured over time as described under “Experimental Procedures.” The rate of receptor degradation is similar for wild-type and mutant AChR (n = 5). Thus, higher surface levels of βK353L/δK351L-AChR stem from increased trafficking to the cell surface rather than decreased turnover.