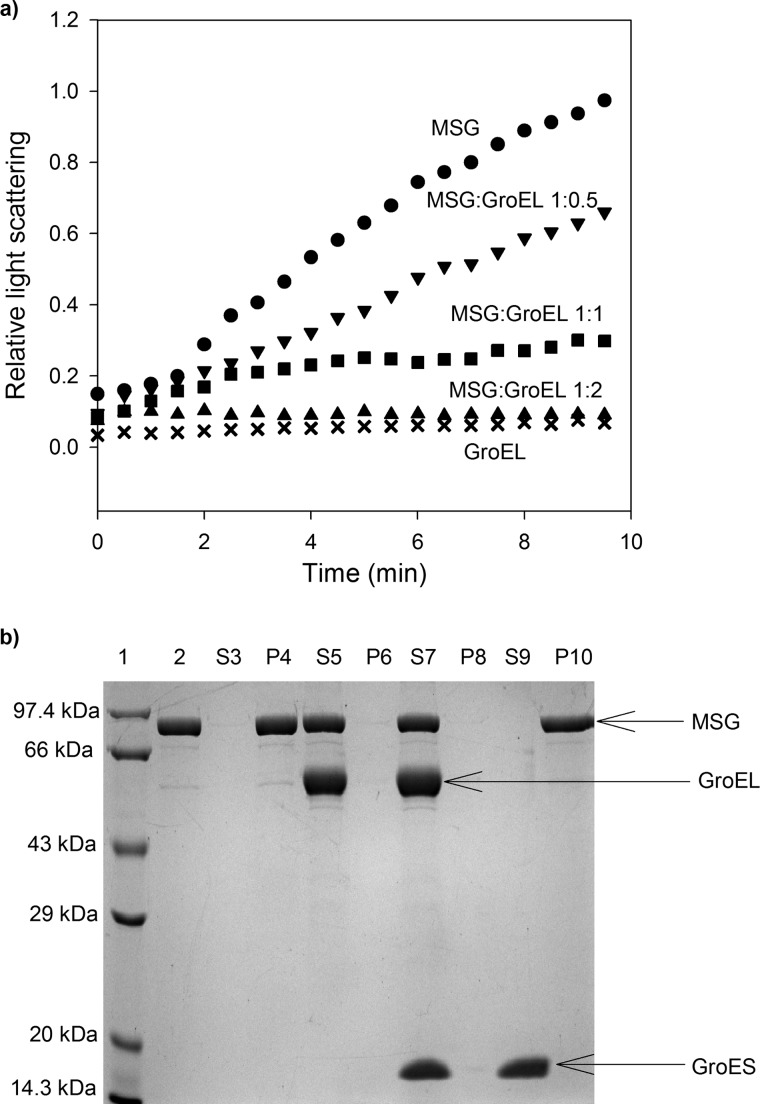
FIGURE 1.
Prevention of thermal aggregation of MSG by GroEL. Aggregation of MSG was monitored by light scattering and SDS-PAGE analysis of supernatant-pellet fractions. a, native MSG at a concentration of 1 μm was incubated in the absence (●) and in the presence of different molar ratios of GroEL in 20 mm Tris buffer, pH 7.8, 10 mm MgCl2, 10 mm KCl at 55 °C. ▾, 0.5:1 GroEL/MSG; ■, 1:1 GroEL/MSG; ▴, 2:1 GroEL/MSG; ×, GroEL only. Aggregation kinetics was monitored by light scattering at 500 nm and normalized with the highest value. b, SDS-PAGE showing supernatant (S)-pellet (P) fractions of heat-treated MSG with and without chaperones. MSG at a concentration of 1 μm was incubated with 2 μm GroEL at 55 °C for 10 min and centrifuged, and supernatant-pellet fractions were run on a 12% SDS gel after normalization. When present, GroES was added in a 2:1 molar ratio to GroEL. Lane 1, protein molecular mass marker; lane 2, native MSG; lane 3, supernatant fraction of heat-treated MSG in the absence of GroEL (S3); lane 4, pellet fraction of S3; lane 5, supernatant fraction of heat-treated MSG in the presence of GroEL (S5); lane 6, pellet fraction of S5; lane 7, supernatant fraction of heat-treated MSG in the presence of GroEL/ES (S7); lane 8, pellet fraction of S7; lane 9, supernatant fraction of heat-treated MSG in the presence of only GroES (S9); lane 10, pellet fraction of S9.