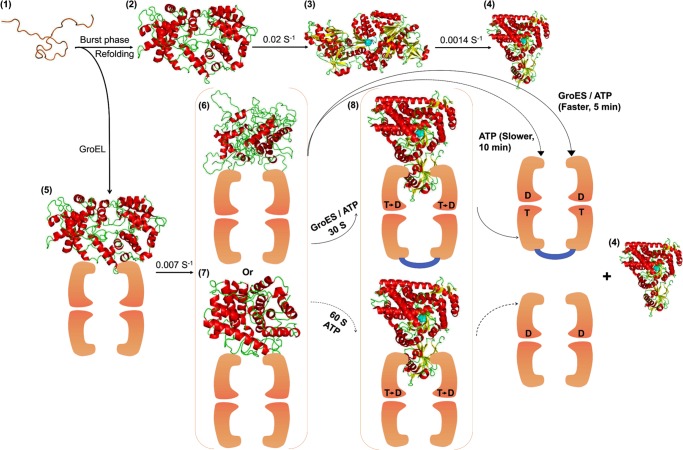
FIGURE 10.
GroEL/GroES-assisted folding model of MSG. The burst phase intermediate of MSG, C (2), is captured by GroEL (orange colored) upon dilution from GdnHCl-denatured MSG (1) to form GroEL-MSG complex, C-G (5). This binding induces minor structural rearrangements in C-G at a slow rate to give rise to a more folding-compatible state, I-G (6 and 7); I-G could be more unfolded (6) or more folded (7) than C-G. Further addition of GroES/ATP or ATP releases the GroEL-bound form of MSG (I-G), which folds to the native state (4) via formation of a compact intermediate, IC-G (8), that is structurally quite close to the native MSG. GroES (shown in blue) binds in trans to the folding polypeptide and doubles the ATP-dependent reactivation rate. Spontaneous refolding (1–4) proceeds through the functional intermediate, IN (3), of which conversion to the native MSG (4) is the slowest step in the MSG refolding pathway. GroEL-mediated folding averts this slowest kinetic phase by channeling the burst phase intermediate of MSG, C (2), to a different folding route (5–8). T and D represent ATP and ADP, respectively. The active site is depicted in cyan. The intermediate IC-G (8) is the most compact of all the intermediates.