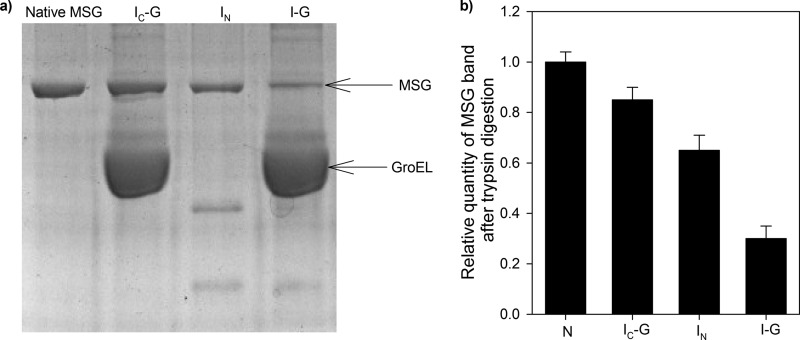
FIGURE 6.
Trypsin digestion assay. Various conformations of MSG were tested by trypsin digestion assay. GroEL-MSG complex was prepared by diluting denatured MSG in GroEL-containing buffer in a 1:4 ratio to GroEL and incubating for 10 min at 25 °C and then dividing it into two parts. The first sample was treated with trypsin 60 s after ATP addition, and in the second sample, no ATP was added before trypsin addition. ATP-mediated reactivation of MSG was stopped after 60 s (IC-G) by addition of 10 mm EDTA following which trypsin was added. For sample without GroEL, trypsin was added 5 min after initiation of refolding (IN). Trypsin treatment was performed at 37 °C for 15 min using a 1:100 (w/w) ratio of trypsin to MSG. Native MSG was used as a control and was treated with trypsin in the same way. Trypsin digestion was stopped by boiling the samples in SDS loading dye for 5 min. a, 10% SDS-PAGE gel showing trypsin-digested mixtures. Lane 1, native MSG; lane 2, IC-G; lane 3, IN; lane 4, GroEL-bound MSG (I-G). b, histogram showing relative quantities of MSG band in each case. Band density was taken as a measure of proteolytic resistance of MSG. The same amount of MSG was loaded in all the lanes so its corresponding band intensities in each of the lanes can be directly compared with each other. Error bars represent the spread of measurements from three separate experiments.