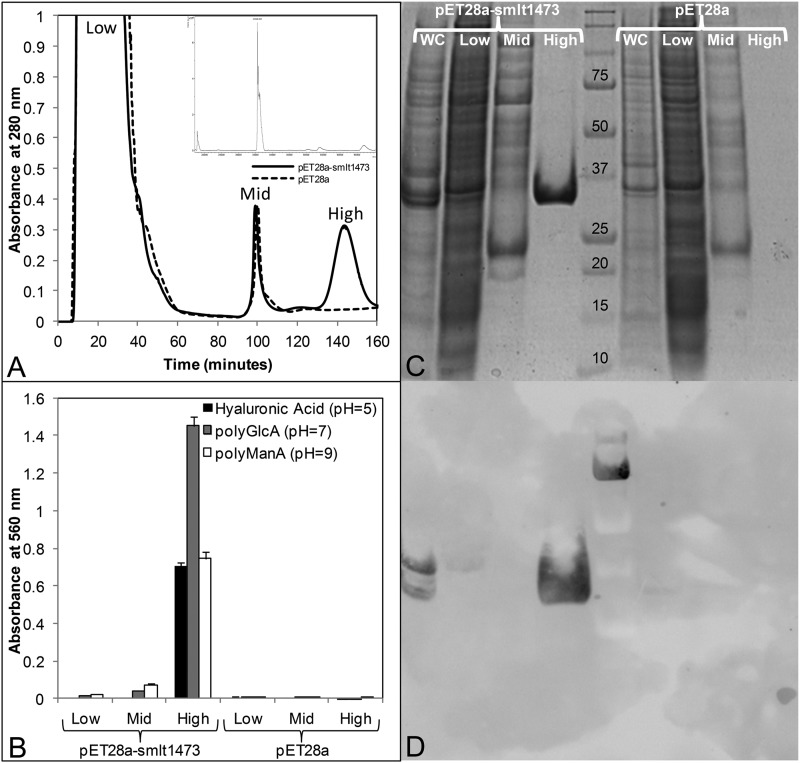
FIGURE 3.
Heterologous expression and one-step purification of Smlt1473 by immobilized metal ion affinity chromatography. A, chromatogram of IMAC purification. Cell lysates prepared from E. coli BL21 cells transformed with pET28a-smlt1473 or pET28a vector and induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside at 18 °C, 200 rpm, were added to Ni2+-bound chelating Sepharose column at t = 0 min and a flow rate of 1.8 ml/min. At t = 70 min a linear imidazole gradient was applied, increasing the imidazole concentration 2.5 mm per minute. Low (∼10 mm), mid (∼85 mm), and high (∼200 mm) imidazole fractions were collected for both pET28a-smlt1473 and pET28a samples. B, fractions were analyzed for lyase activity by mixing 2 μl of sample with 18 μl of 1 mg/ml of HA, poly-GlcA, or poly-ManA in 30 mm buffer at pH 5, 7, or 9, respectively. Oligosaccharide products formed via lyase enzymatic activity were detected by the TBA method (20). All reactions were performed in triplicate and error is reported as S.D. C, SDS-PAGE analysis of each fraction. WC corresponds to whole cell lysate prior to IMAC purification. D, anti-His6 tag Western blot of each fraction.