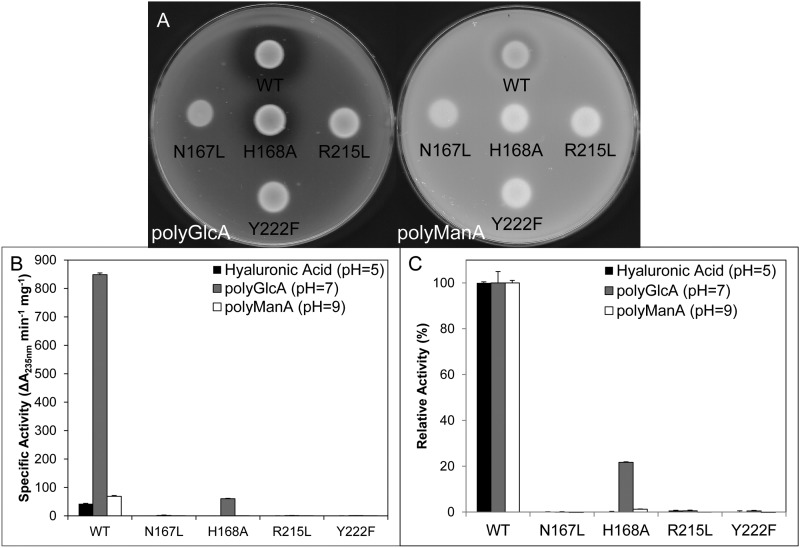
FIGURE 7.
Mutagenesis of predicted catalytic residues. A, E. coli BL21 cultures expressing Smlt1473 WT, N167L, H168A, R215L, and Y222F were dotted onto LB plates solidified with 1% agarose and supplemented with 50 μg/ml of kanamycin and 1 mg/ml of poly-GlcA (left) or poly-ManA (right). A clearing zone is indicative of lyase activity. B, specific activity of Smlt1473 WT and catalytic mutants against HA, poly-GlcA, and poly-ManA at their respective optimal pH values. Enzyme activity was monitored by absorbance at 235 nm. C, TBA confirmation of activity of Smlt1473 WT and putative catalytic mutants against HA, poly-GlcA, and poly-ManA at their respective optimal pH values. 100% activity was taken as the activity of WT against each substrate. All reactions were performed in triplicate and error is reported as S.D.