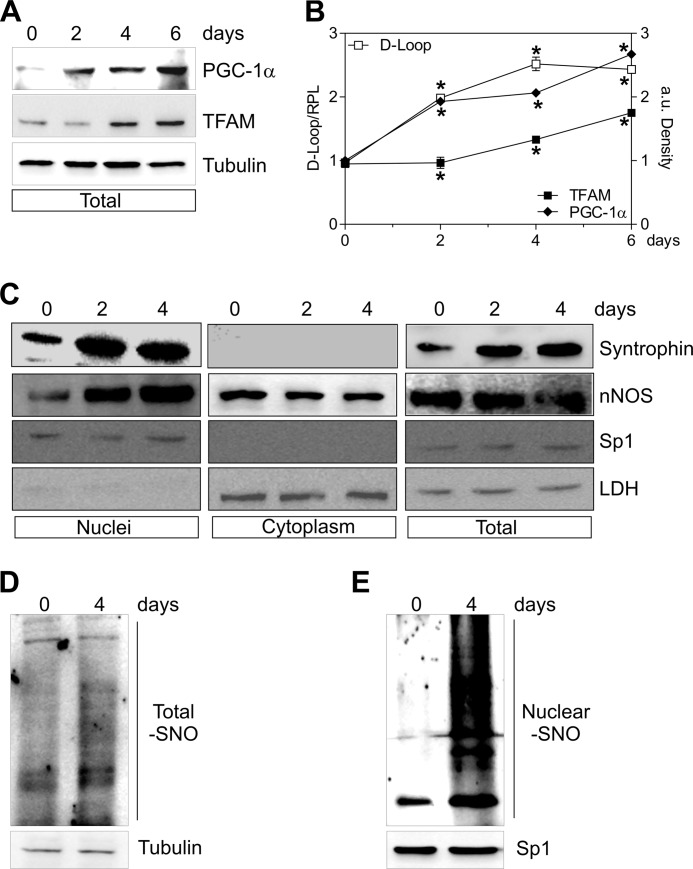
FIGURE 6.
nNOS and α-Syntrophin are recruited to nuclei during C2C12 differentiation. C2C12 cells were differentiated for 0, 2, 4, and 6 days. A, 20 μg of total protein extracts were loaded for detection of PGC-1α and TFAM by Western blot. Tubulin was used as loading control. B, open symbols, DNA was extracted, and relative mtDNA content was assayed by analyzing D-loop level through qPCR. Filled symbols, the increase of PGC-1α (♦) and TFAM (■) was quantified by densitometric analysis of the immunoreactive bands. The data are reported as D-loop/RPL or PGC-1α/Tubulin and TFAM/Tubulin. The data are expressed as means ± S.D. (n = 4; *, p < 0.001 versus day 0). C, 20 μg of nuclear, cytoplasmic, and total protein extracts were loaded for detection of nNOS (using N terminus antibody) and α-Syntrophin by Western blot. Sp1 and LDH were used for assaying the purity of fractions and/or as loading controls. D and E, 500 μg of total (D) and nuclear (E) protein extracts were subjected to S-NO derivatization with biotin. After Western blot, biotin adducts were identified by incubating nitrocellulose membrane with HRP conjugate streptavidin. β-Tubulin and Sp1 were used as loading controls. Immunoblots reported are representative of at least four experiments that gave similar results.