FIGURE 5.
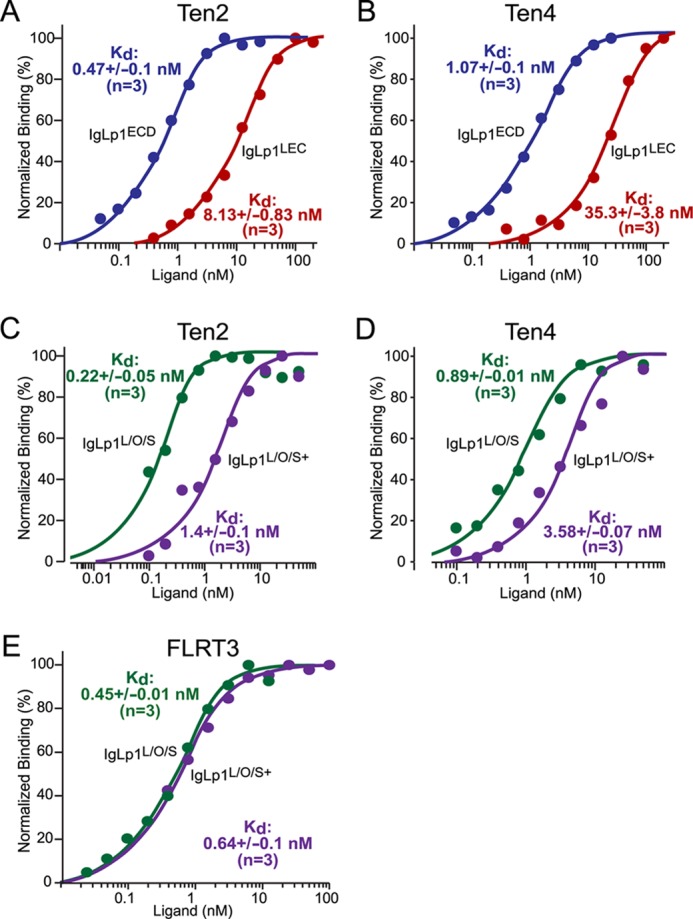
Binding affinity of Lphn1 to Ten2 and Ten4 and to FLRT3 and effect of alternative splicing of latrophilin-1 on such binding. HEK293T cells expressing Ten2, Ten4, or FLRT3 were used to measure the binding affinity of Ig fusion proteins containing the indicated extracellular Lphn1 domains. All data shown are binding after subtraction of the signal obtained with mock-transfected cells included in all assays. Binding data shown are representative graphs of experiments that were conducted at least three times. Data were fit to a Scatchard equation using SigmaPlot software and assuming uniform binding sites; Kd numbers displayed in the graphs show the averages determined in at least three independent experiments. A–E, binding data for the entire Lphn1 extracellular domains (IgLp1ECD; A and B), the N-terminal Lphn1 lectin domain only (IgLp1LEC; A and B), or the N-terminal three Lphn1 domains (i.e. its lectin, olfactomedin-like, and serine/threonine-rich domains); for the latter, splice variants lacking an insert in SSA (IgLp1L/O/S; C–E) or containing such insert (IgLp1L/O/S+; C–E) were analyzed.
