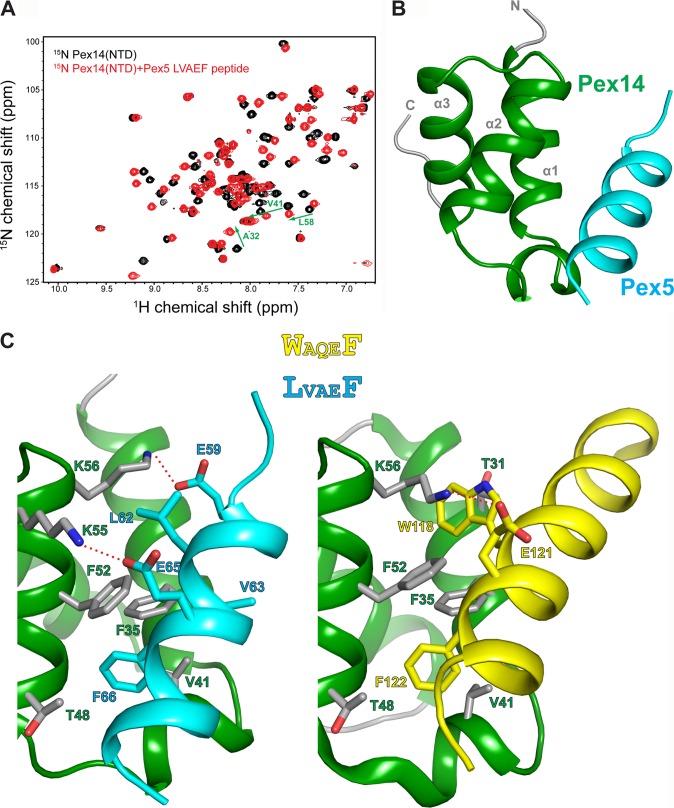
FIGURE 3.
NMR structure of the Pex14-NTD-Pex5-(57–71) complex. A, 1H,15N HSQC spectra of 15N-Pex14-NTD, free (black), and titrated with 2-fold excess of Pex5-(57–71) (red). B, ribbon representation of the lowest energy NMR structure of the Pex14-NTD·Pex5-(57–71) complex. C, structural comparison of Pex14 interaction with LVAEF (left) and WAQEF (right) peptide ligands of Pex5. Side chains of residues involved in intermolecular interactions are shown as sticks. The recognition of Phe (in LVAEF and WAQEF) by Pex14 is similar in the two structures; however, the main difference lies in the recognition of LV (in LVAEF) and Trp (in WAQEF) residues. Plausible salt bridges between Glu and Lys side chains are indicated by red dashed lines in both structures.