FIGURE 8.
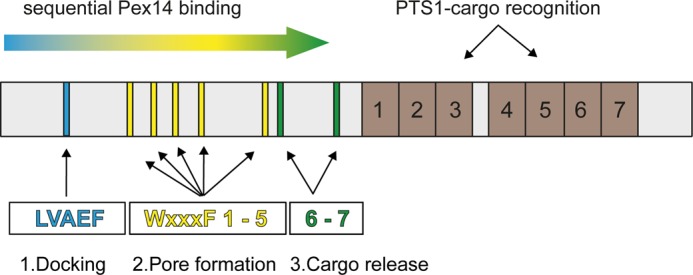
Sliding model of the Pex5-Pex14 interaction. Although PTS1-containing peroxisomal matrix proteins bind to the C-terminal seven tetratricopeptide repeat domains of Pex5 (brown boxes, 1–7), Pex14 interacts via its N-terminal domain with eight penta-peptide motifs located in the N-terminal half of the long isoform of human Pex5 (cyan, yellow and green bars). 1, N-terminal LVXEF motif (LVAEF, cyan) is supposed to make the first contact to Pex14 and serves as the initial membrane docking site of the receptor-cargo complex. 2, upon dissociation, Pex14 can associate with one of the adjacent WXXX(F/Y) motifs W1 to W5, and the novel LVXEF motif can recruit another Pex14. Sequential uploading of W1 to W5 correlates with structural changes of the Pex5-cargo·Pex14 complex and may result in the formation of a transient protein conducting channel in the peroxisomal membrane. 3, finally, interaction of the WXXX(F/Y) motifs W6 and W7 with Pex14-NTD enables cargo release into the peroxisomal matrix.
