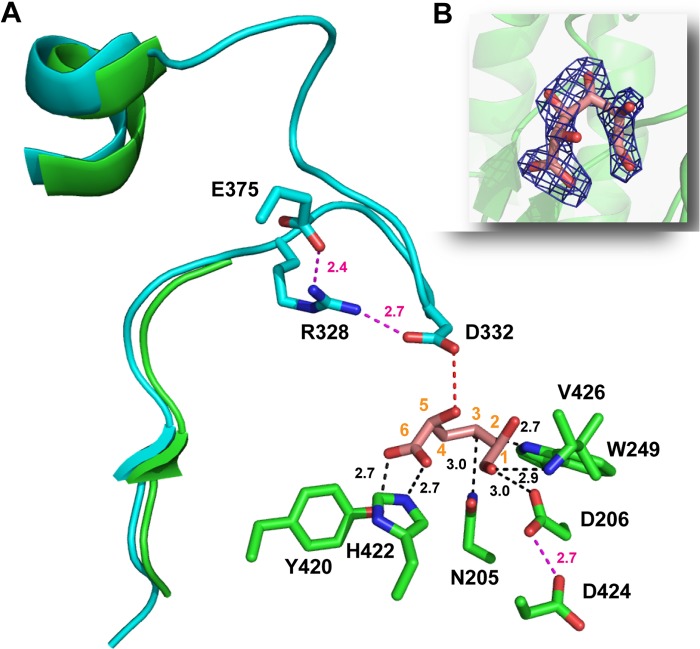
FIGURE 6.
The active site of BoAgu115A. Panel A shows the three-dimensional position of the amino acids (carbons colored green in the structure of the BoAgu115A-GlcA complex and cyan in the apo structure) of BoAgu115A that make polar interactions (indicated by black dotted lines) with bound GlcA (shown in stick format with carbons numbered and colored salmon pink) in its open ring conformation, or polar side contacts with other amino acids (indicated by magenta dotted lines). The distance in Å of these interactions are indicated. The red dotted line is a potential polar contact between Asp-332 and GlcA. The three-dimensional position of Arg-328, Asp-332, and Glu-375 are derived only from the apo structure of BoAgu115A as the loop containing the aspartate and arginine and the side chain of the glutamate are disordered in the BoAgu115-GlcA complex. The secondary structural elements shown in green and cyan, correspond to residues Glu-320 to Glu-345 of BoAgu115A-GlcA and apo BoAgu115A, respectively. The selected amino acids are shown in stick format with the carbons colored green (BoAgu115A-GlcA) or cyan (apo BoAgu115A) with all oxygens and nitrogens colored red and blue, respectively. Panel B shows the electron density map (2Fo − Fc) of GlcA at 1.3 σ. The electron density is shown in dark blue, protomer 1 is displays as a schematic in green and the atoms are colored as described in panel A.