FIGURE 9.
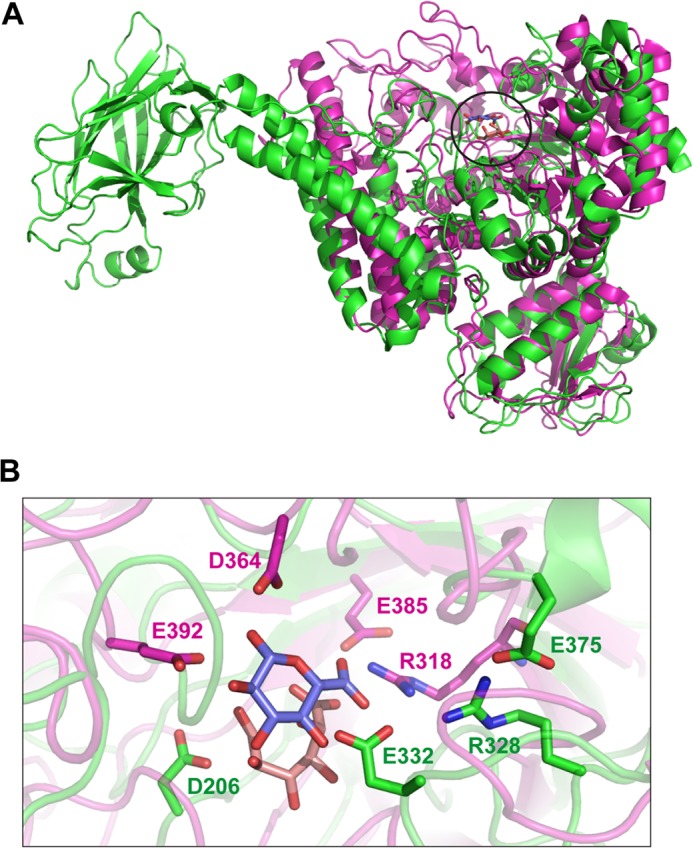
Comparison of the active site of BoAgu115A with a GH67 α-glucuronidase. Panel A shows an overlay of a protomer of BoAgu115A (green) with AguA, a GH67 α-glucuronidase (magenta; PDB code 1MQQ) from G. stearothermophilus (11). The position of the bound GlcA from BoAgu115A and AguA are circled. Panel B shows an overlay of the active site of the two enzymes depicted in panel A in schematic format with the key catalytic and sugar binding residues displayed as sticks. The residues in which the carbons are colored green (apo form of BoAgu115A; see Fig. 6 for the rationale for showing residues from the two structures) are from the GH115 enzyme and amino acids with carbons shown in magenta from the GH67 glucuronidase. The carbons of the GlcA in complex with BoAgu115A and the GlcA bound to AguA are shown in salmon pink and slate blue, respectively, in both panels. All oxygens and nitrogens are shown in red and dark blue, respectively.
