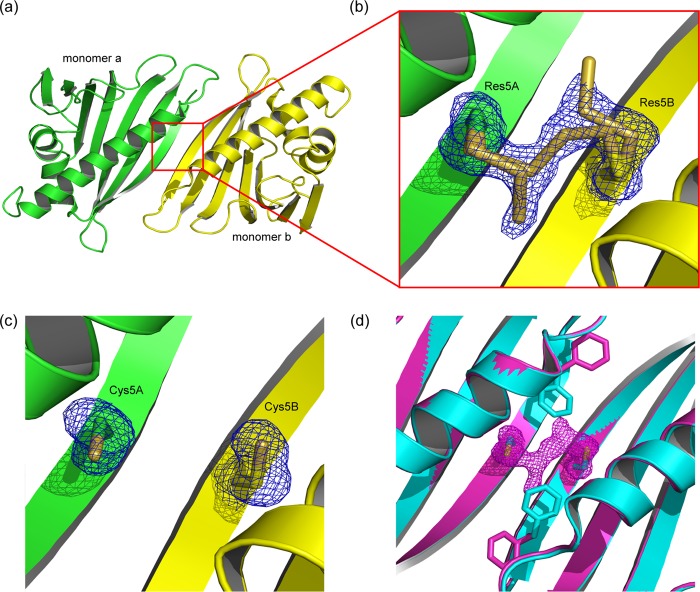
FIGURE 2.
Tetrasulfide dimer interface in the absence and presence of reducing agent. a, cartoon representation of the assembly in the tetrasulfide dimer. The β1-strands of the monomers (green and yellow) elongate the antiparallel β-sheet over the whole quaternary structure. b, close-up view of the dimer interface. The tetrasulfide bridge exhibits partial breaks between SγA-SδA and SδB-SγB. c, the dimer interface after reduction, with the linker electron density vanished. The residual electron density at residue 5 fits cysteines. d, comparison of Phe3 rotamers in the intact (magenta) and reduced (cyan) tetrasulfide dimer. Reduction leads to an approximately 120° flip of Phe3 toward residue 5. The presence of the linker (indicated by its density) keeps Phe3 in the same conformation as observed in the WT structure.