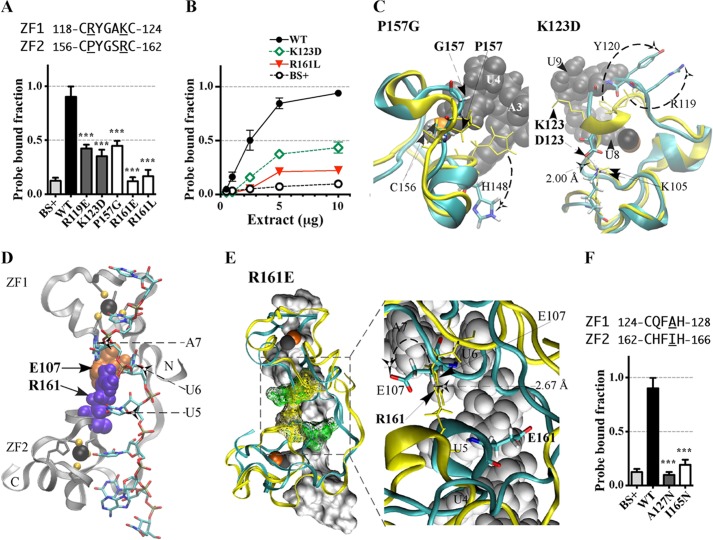
FIGURE 4.
Effects of substitution mutants in the CX5CX3H intervals of the TTP TZF domain on RNA binding. The binding of protein to RNA is expressed as the probe-bound fraction. Above the graphs are the amino acid sequences of the intervals under study. The underlined residues are those that were mutated and whose effects on binding are shown in the bar graphs. The results from mutants of finger 1 are shown as gray columns, and those of finger 2 are shown as white columns. A, substitution mutants in the CX5C intervals. The results (mean ± S.D. (error bars)) are from four similar gel shift assays using 0.2 nm probe TARE5. B, binding assays with 0.2 nm TARE5 probe in serially diluted extracts, with total protein ranging from 10 to 1 μg. The probe-bound fractions (mean ± S.D.) are from four similar gel shift assays. C, superposition of the structural ensembles of finger 2 of WT TTP and mutant P157G (the RMSD is 10.37 Å; RMSD values were calculated from the superposition of the TZF domains of each mutant TTP with RNA-bound WT TTP) or finger 1 of WT TTP and mutant K123D (RMSD is 2.86 Å). The ribbon diagram of the WT TTP peptide backbone and side chains (sticks) are in yellow, the ribbon diagram of the mutant peptide backbone is in cyan, and side chains are shown in colors. RNA nucleosides are shown as gray spheres. Zinc atoms are shown as silver and copper (in P157G) or black and copper (in K123D) spheres. D, ribbon diagram of the peptide backbone of the human TTP TZF domain in complex with the ARE nonamer (sticks). The side chains of Glu107 (orange spheres, at the lead-in to finger 1) and Arg161 (purple spheres, at the C+5 position of the finger 2 CX5C region) are shown. Dashed arrows indicate nucleosides U5, U6, and A7 that interact with Glu107 and Arg161. Zinc atoms (black spheres) and the zinc-coordinating residues (ball and stick) of each finger are also displayed. E, superposition of the peptide backbone of the TZF domains of WT TTP (yellow ribbon) in complex with the ARE nonamer (surface representation) and the mutant R161E (cyan ribbon; RMSD is 4.66 Å). Zinc atoms are shown as black and copper spheres. Residues Glu107 and Arg161 (yellow mesh in the WT TZF domain) and Glu107 and Glu161 (green mesh in the mutant R161E) are shown (left). The right panel depicts the detailed view of the interaction between Arg161 and Glu107 (yellow sticks) in the WT and the non-interacting Glu107 and Glu161 (color sticks) in the mutant R161E. RNA nucleosides U4, U5, U6, and A7 (gray spheres) are indicated. F, substitution mutants in the CX3H intervals. The results (mean ± S.D.) are from four similar gel shift assays.