TABLE 1.
Residues that form the ARE base binding pockets in the TTP and ZFP36L2 TZF domains
The composition of the binding pockets for each RNA base of the RNA oligomer (1-UUAUUUAUU-9) was obtained from the TTP simulation solution model, with the equivalent residue in parentheses from the NMR structure of the TZF domain of ZFP36L2 (TIS11d; PDB code 1RGO) in complex with RNA. Binding pockets are made up largely of residues from equivalent sequence positions within the TZF domain of the two structures. The TZF domain of human TTP is comprised of residues 103–166 from GenBankTM accession number NP_003398.13; the corresponding residues of human ZFP36L2 (Tis11D) are 153–216 of NP_008818.3.
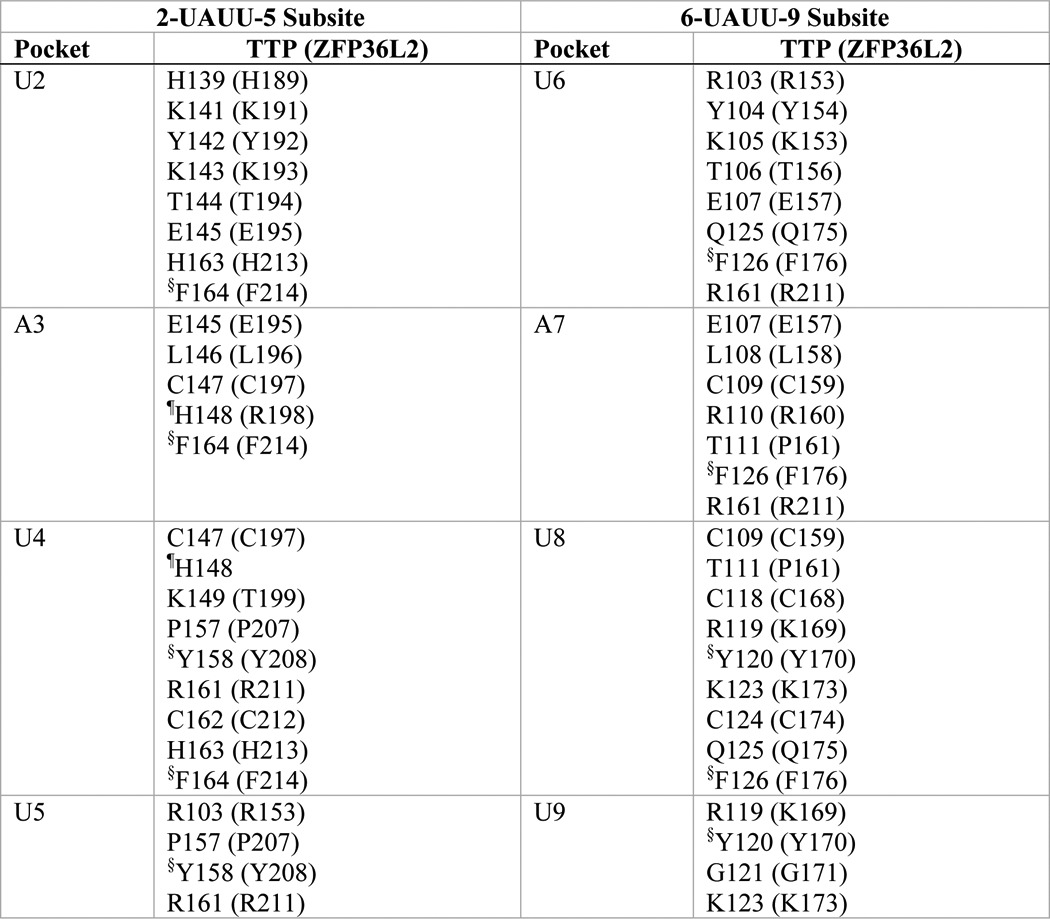
§ Side chain involved in stacking with the RNA bases: U2-Phe164-A3; U4-Tyr158-U5; U6-Phe126-A7; U8-Tyr120-U9 (in ZFP36L2: U2-Phe214-A3; U4-Tyr208-U5; U6-Phe176-A7; U8-Tyr170-U9).
¶ His148 of TTP is located at the rim of both A3 and U4 pockets. Arg198 of ZFP36L2 forms part of the wall of the pocket for A3.
