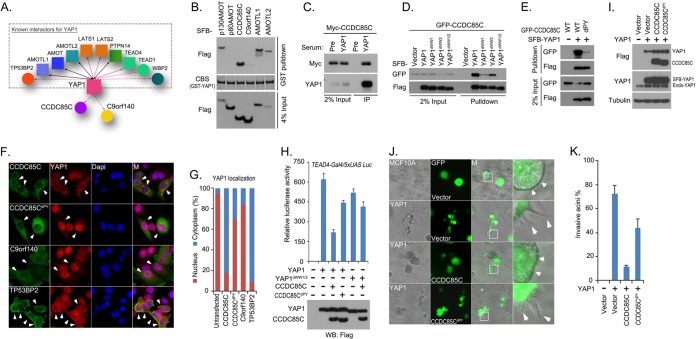
Fig. 5.
Analysis of YAP1 interactome suggests that CCDC85C is a new regulator of YAP1. A, Schematic illustration of major YAP1-interacting proteins. YAP1 was also identified in the bait protein AMOT-, AMOTL1-, AMOTL2- or PTPN14-containing protein complexes and therefore they are indicated by solid black lines with double arrows. Bait proteins TEAD4, LATS1, and LATS2 are indicated in the YAP1 interactome by a solid line with a single arrow, while other prey proteins are linked to YAP1 via a dashed line with a single arrow. The reported YAP1-binding proteins in this interactome are grouped in a dashed square; two previously unknown prey proteins, CCDC85C and C9orf140, were left outside of the square. B, CCDC85C is a newly discovered YAP-associated protein. Bacterially expressed and purified GST-YAP1 fusion protein was used to pull down exogenously expressed SFB-tagged p130AMOT, p80AMOT, CCDC85C, C9orf140, AMOTL1, and AMOTL2 in vitro. CBS, coomassie blue stain. C, Association of endogenous YAP1and Myc-tagged CCDC85C was confirmed by a co-immunoprecipitation experiment. Immunoprecipitation (IP) assay was performed using 293T cell extracts and anti-YAP1 serum. Pre-immune serum was used as the control. Immunoblotting was performed with indicated anti-YAP1 anti-serum or anti-Myc antibody. D, Two WW domains of YAP1 were required for its interaction with CCDC85C. 293T cells were transfected with constructs encoding GFP-tagged CCDC85C together with constructs encoding SFB-tagged wild-type or indicated WW domain-deletion mutants of YAP1. Pull-down experiments were carried out using S-protein beads, and immunoblotting was performed with indicated antibodies recognizing GFP or Flag-tag. E, The PY motif of CCDC85C was required for its interaction with YAP1. 293T cells were transfected with a construct encoding GFP-tagged wild-type or indicated CCDC85C PY motif-deleted mutant together with a construct encoding SFB-YAP1. Pull-down experiments were carried out using S-protein beads and immunoblotting was performed with indicated antibodies recognizing GFP or Flag-tag. F, G, CCDC85C translocated YAP1 from nucleus into cytoplasm. The localization of endogenous YAP1 was detected by anti-YAP1 antibody in HeLa cells expressing indicated SFB-tagged wild-type or PY motif deleted CCDC85C mutant, C9orf140, or TP53BP2. The localization of indicated SFB-tagged proteins was detected with anti-Flag antibody. Nuclei were stained by DAPI. M stands for merged. The percentages of nuclear and cytoplasmic YAP1 localization are quantified in (G). H, CCDC85C inhibited YAP1 transactivation activity in a luciferase reporter assay. Luciferase reporter assay was performed by cotransfecting indicated YAP1 or YAP1 two WW domain-deleted mutant with indicated CCDC85C or its PY motif-deleted mutant in 293T cells. Firefly Renilla was used as the internal control. Data are presented as mean ± s.d. from three different experiments. The transfected proteins were detected by Western blotting as shown at the bottom of the panel. I, Overexpression of CCDC85C or its PY motif -deleted mutant in MCF10A cells overexpressing YAP1. MCF10A cells expressing SFB-YAP1 were transduced with viral particles encoding SFB-tagged CCDC85C or its PY motif-deleted mutant. This lentiviral vector contains a separate promoter that controls the expression of GFP. Exogenously expressed YAP1 and CCDC85C were detected by anti-Flag antibody, whereas endogenous and exogenous YAP1 were detected by anti-YAP1 antibody. J, CCDC85C suppressed invasive acini formation by MCF10A cells overexpressing YAP1. MCF10A cells in (I) were subjected to 3D culture in Matrigel. GFP indicated cells positively transduced with control virus or viral particles expressing wild-type CCDC85C or its PY motif-deleted mutant. White squares are enlarged in the far right column. Small white arrows indicate normal acini morphology and large white arrows indicate invasive acini morphology. K, The percentages of invasive acini shown in (J) were quantified.