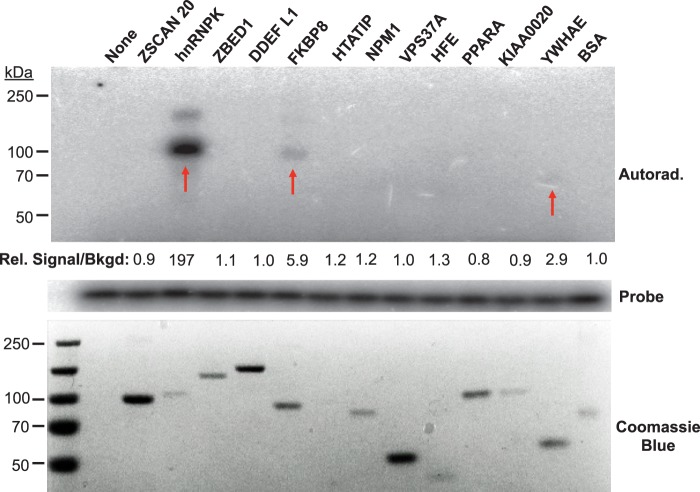
Fig. 2.
Biochemical validation of the HCV SL1-host protein interactions identified in the human proteome chips. The 12 protein hits from the SL1 screen were purified as GST fusions from yeast (16), and were subjected to UV-crosslinking assays with HCV SL1. The proteins were separated on SDS-PAGE and HCV SL1-bound proteins were detected by autoradiography (upper panel). The lower panel indicates the input proteins in the EMSA. The amount of probe presented in each reaction is shown in the slice of the autoradiogram in the middle of the figure. Among the proteins of interest, hnRNP K, FKBP8, and YWHAE showed the binding affinity to HCV SL1 in the crosslinking analysis as indicated by red arrows. The signal intensities of the radiolabeled EMSA bands and the Coomassie blue stained protein bands were quantified using the Image Quant 5.2 software and the ratio of the signal was normalized to that of BSA.