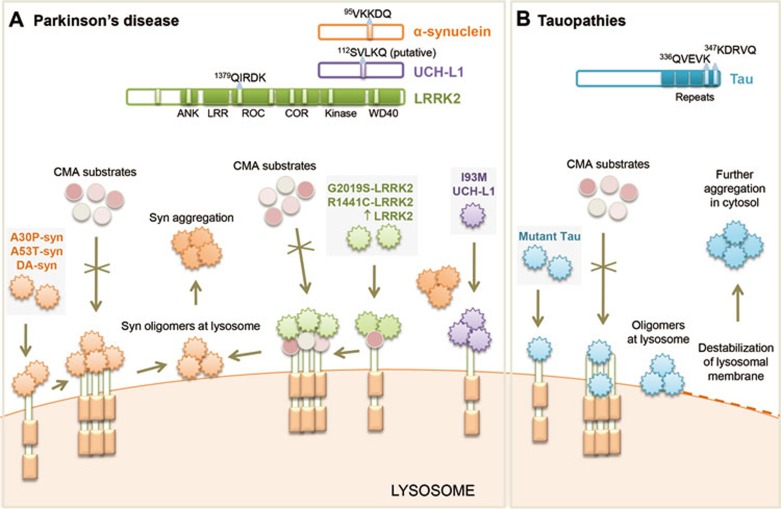
Figure 2.
Impairment of CMA by pathogenic proteins contributes to neurodegeneration. (A) Mechanisms of CMA failure in Parkinson's disease (PD). Many PD-related proteins bear CMA-targeting motifs (α-synuclein, UCH-LI and LRRK2 shown here) (top). LRRK2 has eight CMA-targeting motifs but only the sequence of the most commonly used is shown. Both wild-type α-synuclein and LRRK2 are degraded by CMA. Mutant forms of these proteins and of UCH-L1 bind abnormally to LAMP-2A, albeit via different mechanisms, leading to blockage of their own degradation as well as degradation of other CMA substrates. Dopamine-modified α-synuclein and abnormally high levels of wild-type LRRK2 also impair CMA. Failure of CMA causes accumulation and aggregation of these toxic proteins that could contribute to Lewy body formation in PD. Alterations of CMA by mutant LRRK2 and UCH-L1 show converging toxic effects on α-synuclein aggregation. (B) Perturbation of CMA by mutant tau in tauopathies. wild-type tau protein is a bona fide CMA substrate carrying two CMA-targeting motifs (top). Pathogenic variants of tau fail to translocate fully into the lysosomal lumen. Such inefficient translocation promotes partial cleavage of tau and formation of tau oligomers at the lysosomal membrane resulting in destabilization of lysosomal membrane and lysosomal leakage. Release of lysosomal tau oligomers into the cytosol may act as a precursor for further tau aggregation.