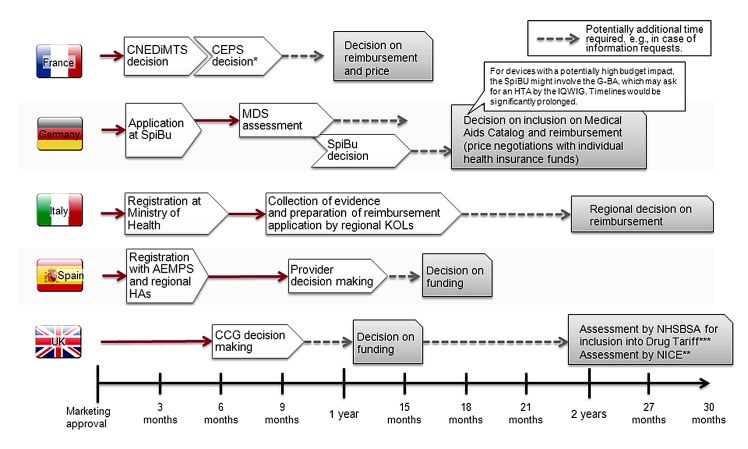
Figure 1.
Reimbursement processes and typical timelines for innovative devices. *Products asking for Amélioration du Service Médical Rendu I, II, or III in their evaluation dossier will have to submit health–economic data to the health economics committee in the health authorities, reporting to the Comité Economique des Produits de Santé. **Depending on budget impact and or/usage on local level. ***Inclusion in drug tariff is a prerequisite for broad funding but may happen only some time after launch. CNEDiMTS, Commission Nationale d’Évaluation des Dispositifs Médicaux; CEPS, Comité Economique des Produits de Santé; SpiBu, Spitzenverband Bund/GKV Spitzenverband; MDS, Medizinischer Dienstdes Spitzenverbandes Bund der Krankenkassen; IQWiG Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen; G-BA, Gemeinsamer Bundesausschuss; AEMPS, Agencia Española de Medicamentos y Productos Sanitarios; HAs, health authorities; CCG, clinical commissioning group; NHSBSA, NHS Business Service Authority.