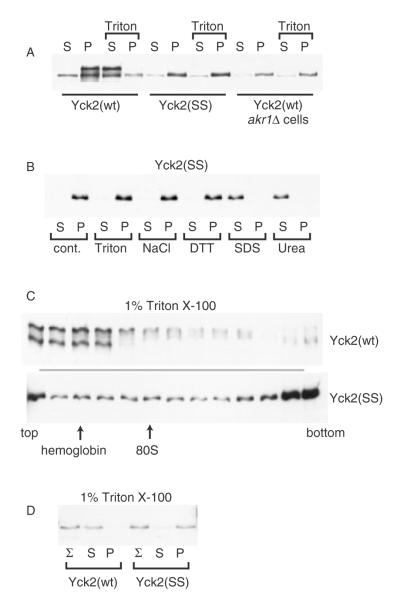
Figure 1.
Non-palmitoylated Yck2 from glass bead lysates fractionates as a part of a high molecular-weight aggregate. Glass bead lysates prepared from either wild-type or akr1Δ yeast cells, expressing either Yck2(wt) or the palmitoylation-deficient Yck2(SS) mutant, were fractionated by centrifugation. The expressed Yck2 proteins were all N-terminally tagged with the 6× His/FLAG/HA tri-tag, allowing Western blot detection with anti-HA-HRP. (A) Lysates from cells expressing the indicated Yck2 proteins from GAL1 promoter (2 h of expression from CEN/ARS plasmid-borne alleles) were treated with a 30 min, 4 °C membrane solubilizing incubation with 1% Triton X-100 or a parallel mock solubilization (no detergent) to high-speed centrifugation, to yield the supernatant (S) or pellet (P) fractions, which were analysed by anti-HA Western blotting. (B) Glass bead lysates deriving from cells expressing Yck2(SS) from the GAL1 promoter were subjected to 30 min treatments with either 1% Triton X-100, 1 m NaCl, 10 mm DTT, 1% SDS or 6 m urea (incubations were at 4 °C except for SDS and DTT, which were at 23 °C), then fractionation by high-speed centrifugation and, finally, anti-HA Western blotting. (C) Lysates from cells expressing either Yck2(wt) or Yck2(SS) from the GAL1 promoter were treated with 1% Triton X-100 prior to sucrose gradient fractionation. Samples of each gradient fraction were analysed by anti-HA Western blotting. The positions of the endogenous 80S ribosome and added haemoglobin are indicated. (D) Detergent-treated lysates prepared from cells expressing either Yck2(wt) or Yck2(SS) from the YCK2 promoter were analysed as for (A), except that, in addition, samples taken just prior to the centrifugal fractionation were also analysed (Σ)