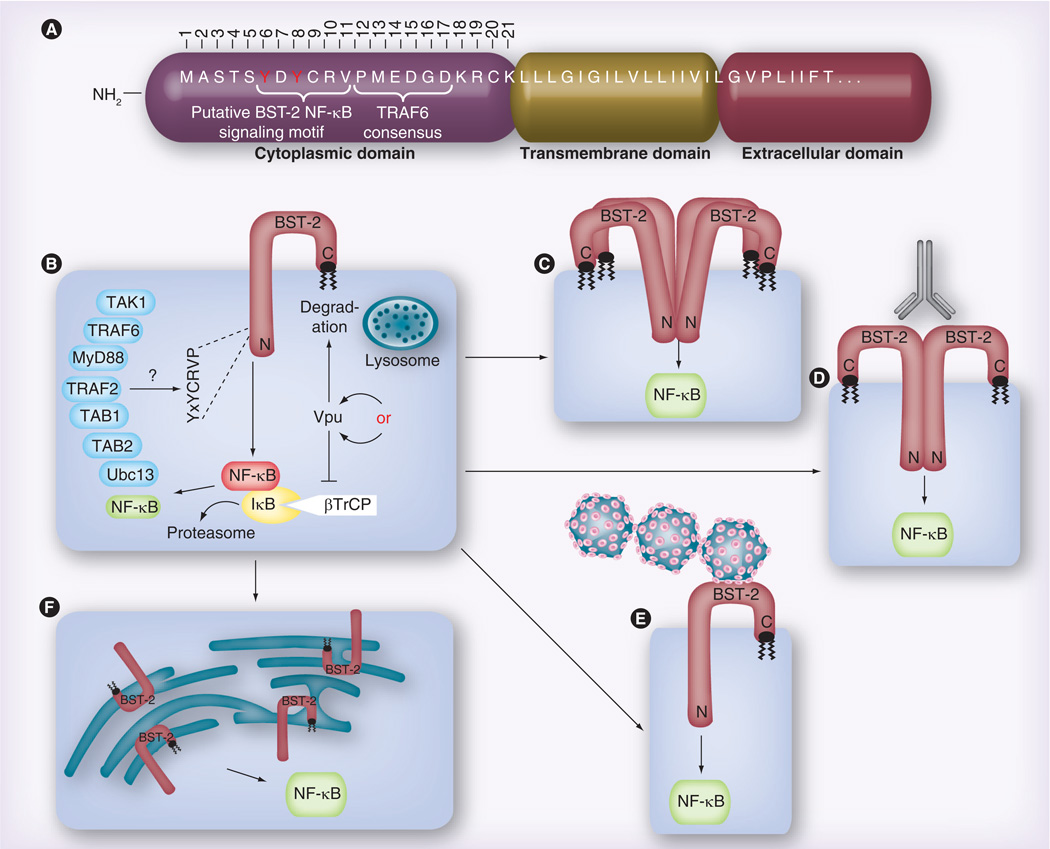
Figure 1. Data and models proposed in the Galão et al. and Tokarev et al. papers regarding the BST-2-dependent activation of NF-κB.
(A) Overview of the putative domain structure for the BST-2 NH2-terminus, including the approximately 21-residue cytoplasmic domain (numbered), the transmembrane domain and the first few residues of the extracellular domain. (B) Both groups demonstrate that, when overexpressed via transient transfection, BST-2 alone can activate NF-κB, and that Vpu expression reduces this activation. Data shown by Tokarev et al. suggest that Vpu accomplishes this via sequestration of the ubiquitin ligase component βTrCP, which promotes NF-κB activity, while Galão et al. assume that Vpu degrades BST-2, thereby eliminating the source of signaling. Both groups also show a role for the BST-2 YxY motif, which was variously observed to be required for binding to TRAF6 (Galão), or TAB1 and TAK1 (Tokarev). The two groups identified partially overlapping sets of other NF-κB signaling intermediates, which were found to be either directly bound to BST-2, or necessary for BST-2 signaling to NF-κB. (C) Tokarev et al. find that BST-2 must be capable of multimerization in order to activate NF-κB, while (D) Galão et al. show that antibody crosslinking of surface-associated BST-2 can further enhance BST-2 activation of NF-κB. (E) Similarly, Galão et al. also find that surface-associated (‘tethered’) virus enhances BST-2-dependent NF-κB activation. (F) Tokarev et al. provide evidence that BST-2-dependent endoplasmic reticulum stress leads to NF-κB activation.