Abstract
Bendamustine demonstrated synergistic efficacy with bortezomib against multiple myeloma (MM) cells in vitro and seems an effective treatment for relapsed-refractory MM (rrMM). This phase II study evaluated bendamustine plus bortezomib and dexamethasone (BVD) administered over six 28-day cycles and then every 56 days for six further cycles in patients with rrMM treated with ⩽4 prior therapies and not refractory to bortezomib. The primary study end point was the overall response rate after four cycles. In total, 75 patients were enrolled, of median age 68 years. All patients had received targeted agents, 83% had 1–2 prior therapies and 33% were refractory to the last treatment. The response rate⩾partial response (PR) was 71.5% (16% complete response, 18.5% very good PR, 37% partial remission). At 12 months of follow-up, median time-to-progression (TTP) was 16.5 months and 1-year overall survival was 78%. According to Cox regression analysis, only prior therapy with bortezomib plus lenalidomide significantly reduced TTP (9 vs 17 months; hazard ratio=4.5; P=0.005). The main severe side effects were thrombocytopenia (30.5%), neutropenia (18.5%), infections (12%), neuropathy (8%) and gastrointestinal and cardiovascular events (both 6.5%). The BVD regimen is feasible, effective and well-tolerated in difficult-to-treat patients with rrMM.
Keywords: bendamustine plus bortezomib and dexamethasone, relapsed-refractory multiple myeloma, overall response rate, overall survival, time-to-progression, safety
Introduction
Multiple myeloma (MM) is one of the most common haematological malignancies and is characterized by multiple relapses, reduced response durations and ultimately treatment refractoriness.1 The introduction of novel agents such as thalidomide, bortezomib and lenalidomide has improved outcomes for patients with newly diagnosed and relapsed-refractory MM (rrMM).2 Immunomodulatory drugs (IMIDs) and proteasome inhibitors have demonstrated effectiveness, as single-agent or combination regimens, in rrMM.1
Factors, including the quality and duration of prior response, residual toxicity, and disease and patient characteristics, influence the choice of treatment for patients with rrMM.3 Although the optimal treatment sequence for patients with rrMM is not clear, retreatment is common following a prolonged response without severe toxicity,4 or if not, class switching is favoured.
Pretreated patients with advanced disease, especially if refractory to available therapies, have a particularly poor prognosis with few options outside of clinical trials.5 Haemato-oncologists are increasingly faced with managing rrMM patients who experience multiple relapses and progressively shorter response durations, even with novel targeted agents.6 There is therefore an urgent need for improved therapeutic options for rrMM patients and for agreement on an optimized treatment sequence that focuses on obtaining the optimal duration of remission first-line and in early lines of treatment to maximize outcomes.
Bortezomib regimens have demonstrated marked activity in rrMM, particularly when combined with dexamethasone or liposomal doxorubicin.7, 8, 9, 10, 11, 12, 13, 14, 15, 16 These combinations are associated with partial remission (PR) rates of 50–70% and median time-to-progression (TTP) of 6–11 months in populations mainly pretreated with alkylators or, to a lesser extent, thalidomide.10, 12, 17, 18, 19, 20
Bendamustine is a hybrid-alkylating agent comprising a purine-like benzimidazole ring and nitrogen mustard group. It has a unique mechanism of action compared with most conventional alkylators, eliciting more extensive and durable DNA damage, resulting in cell death by mitotic catastrophe, higher levels of p53 activation and induction of p53-dependent genes.21, 22, 23 This alternative cell death pathway and potent activation of apoptosis may partially explain bendamustine's effectiveness in alkylator-resistant cells.24 Bendamustine has been successfully used in Hodgkin's disease, follicular and aggressive non-Hodgkin's lymphomas, chronic lymphocytic leukaemia, myelodysplastic syndrome, breast and lung cancer.25 After phase I assessments,26 several trials evaluated bendamustine alone or combined with new drugs in patients with MM.27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Bendamustine demonstrated a predictable and manageable toxicity profile, mainly comprising neutropenia, thrombocytopenia, mucositis and infections. Moreover, its pharmacokinetics were unaffected by impaired renal function, which is common among MM patients. Bendamustine combined with prednisone was also more effective than melphalan–prednisone in front-line MM patients ineligible for transplantation.40 Bendamustine is approved in Europe, but not the United States, for newly diagnosed MM patients aged >65 years with neuropathy who are ineligible for thalidomide or bortezomib. Therefore, most patients with de novo MM continue to be treated with classical alkylators rather than bendamustine. Although seldom used front line, bendamustine shows incomplete cross-resistance with other alkylating agents, making its potential use in salvage therapy particularly attractive.
In preclinical studies, bortezomib enhanced the in vitro sensitivity of MM cells to bendamustine.41 Moreover, bendamustine was more effective in patients washed out from bortezomib by at least 6 months.33 These observations provided the rationale for our study, namely to evaluate bendamustine plus bortezomib and dexamethasone (BVD) in patients with rrMM not refractory to bortezomib and with moderately advanced disease.
A recent phase I study determined the maximum-tolerated dose of bendamustine plus bortezomib without apparent dose-limiting toxicities.37 However, several doses and schedules of bendamustine plus bortezomib had already been used in phase II studies, with variable results and toxicities, before publication of these phase I results.33, 34, 35, 36
The aim of the present phase II study was therefore to assess the efficacy and toxicity of BVD as induction and consolidation therapy in patients with rrMM.
Subjects and methods
Study design and protocol
This prospective, single-arm, open-label, phase II study conducted in 21 Italian centres assessed the efficacy and toxicity of BVD in patients with rrMM not refractory to bortezomib.
The study was approved by the local ethics committees (EudraCT No: 2010-020072-33) and complied with Guidelines for Good Clinical Practice approved by International Conference of Harmonization and the Declaration of Helsinki. All patients provided written informed consent.
Patients received bendamustine (Levact; Mundipharma Pharmaceuticals Ltd) 70 mg/m2 intravenously on days 1 and 8, bortezomib (Velcade; Janssen-Cilag Ltd) 1.3 mg/m2 intravenously . on days 1, 4, 8 and 11 and dexamethasone 20 mg was administered intravenously or orally (per os) on days 1–2, 4–5, 8–9 and 11–12 before bendamustine and bortezomib for the first two cycles. In subsequent cycles, dexamethasone 20 mg was administered intravenously or per os on days 1, 8, 15 and 22 after bendamustine 70 mg/m2 on days 1 and 8 and bortezomib 1.3 mg/m2 on days 1, 8, 15 and 22. Treatment cycles were initially administered every 4 weeks up to four cycles. Patients achieving a response⩽PR were taken off study. Patients experiencing ⩾PR received two additional induction cycles followed by a 12-month consolidation phase with cycles repeated every 2 months. Therefore, patients with a PR after the induction phase could receive up to 18 months of treatment and 12 BVD cycles.
Patients
Eligible patients had: age 18–85 years; Eastern Cooperative Oncology Group performance status ⩽2; life expectancy ⩾3 months; measurable disease (>10 g/l monoclonal gammopathy or >200 mg per day proteinuria); and rrMM after autologous stem cell transplantation (ASCT) or conventional chemotherapy but treated with ⩽4 prior therapies. All previous MM therapies, including radiation, cytostatic therapy and surgery, had to be completed ⩾4 weeks before initiating study treatment without corticosteroid therapy. Eligible patients also had: absolute neutrophil count ⩾1.0 × 109/l; platelet count ⩾75 × 109/l; creatinine clearance >30 ml/h; total bilirubin ⩽1.5 mg/dl; and aspartate aminotransferase/alanine aminotransferase ⩽2 × upper limit of normal or ⩽5 × ULN, if hepatic lesions were present. Eligible patients were free of prior malignancies for ⩾5 years with the exception of curatively treated basal cell, squamous cell carcinoma of the skin or carcinoma in situ of the cervix or breast use of effective contraception was mandatory for fertile patients during, and 6 months after, study treatment.
Patients were excluded if they had: used any other experimental drug or therapy within 28 days of baseline; known hypersensitivity to study drugs; previously received bendamustine; refractoriness to bortezomib; a remission duration <6 months with prior bortezomib regimen; peripheral neuropathy ⩾grade 2; heart disease (New York Heart Association grade III–IV); uncontrolled diabetes or glaucoma; prior allogeneic stem cell transplantation; concurrent use of anticancer treatments not permitted in the treatment plan; known positivity for human immunodeficiency virus, or hepatitis B or C.
Varicella-zoster virus prophylaxis with acyclovir or valacyclovir and of Pneumocystis jirovecii with trimethoprim sulphamethoxazole were mandatory, whereas antibacterial prophylaxis was not. However, after an interim analysis of the first 30 enrolled patients revealed an excess of pneumonia, quinolone prophylaxis was recommended during the first four BVD cycles.
Assessments
The primary end point was achievement of a response ⩾PR (i.e. overall response rate (ORR)) after four BVD cycles according to 2006 International Myeloma Working Group and International Myeloma Foundation, International Uniform Response Criteria for MM.42 Serum and urine samples were collected for M-protein quantification and disease status every 4 weeks during treatment and every 8 weeks during follow-up. Patients were followed until documentation of progressive disease or death. Toxicity was assessed at each treatment and follow-up visit.
Secondary end points included the rate of acute and late toxicity according to National Cancer Institute Common Terminology Criteria for Adverse Events criteria version 3.0 (NCI-CTCAE v.3.0), complete remission (CR) rate, time-to-response, TTP, progression-free survival (PFS) and overall survival (OS). Disease progression and relapse were assessed using the International Myeloma Working Group criteria.42
Dose reductions or interruptions/withdrawals were protocol defined. Treatment was withdrawn for: disease progression; unacceptable toxicity; treatment delay >4 weeks; administration of non-study antineoplastic medication; withdrawn consent; investigator decision; pregnancy/insufficient contraception; and lack of follow-up or death.
Statistical methods
Sample size determinations were based on an expected ⩾60% response rate, as previously observed with bortezomib–dexamethasone.10 Fleming's one-stage design for pilot studies was applied.43 An ORR ⩽40% with experimental therapy was considered inadequate, whereas an ORR ⩾60% signified a promising candidate for phase III development. The probabilities of type I and II errors were 5% and 20%, respectively, corresponding to a power of 80% and required intention-to-treat cohort of ⩾70 patients. The percentage ORR (CR+very good partial response (VGPR)+PR) was calculated along with 95% confidence intervals (CIs). Cumulative dose intensity was calculated as the ratio of expected/administered dose of drug.
Univariate analysis of the association between each covariate and response ⩾PR was performed by χ2 test (or Fisher's exact test). The association between time-dependent variables and covariates was analysed by Cox regression analysis. A hazard ratio was calculated for each variable. All continuous variables were categorized by clinical judgement or empirically (i.e. according to calculated regression coefficients). Maximum-likelihood values were estimated, and variables with P⩽0.1 were included in the multivariate analysis, whereas those with P>0.05 were excluded. Estimated hazard ratios and CIs were calculated for all significant variables. Time-to-response, TTP, PFS and OS were assessed by Kaplan–Meier analysis. Time-to-response was defined as the time from treatment start to first detection of response ⩾PR. PFS was defined as the time from initial dose of chemotherapy to disease progression, death or the date of last assessment without any such event (censored observation). TTP was defined as the time from the initial dose of chemotherapy to relapse/progressive disease or censoring. OS was determined by measuring the time from initial dose of chemotherapy to death or last observation (censored). Statistical analysis was performed using Statistical Package for the Social Sciences software (SPSS, Chicago, IL, USA).
Results
Patient disposition
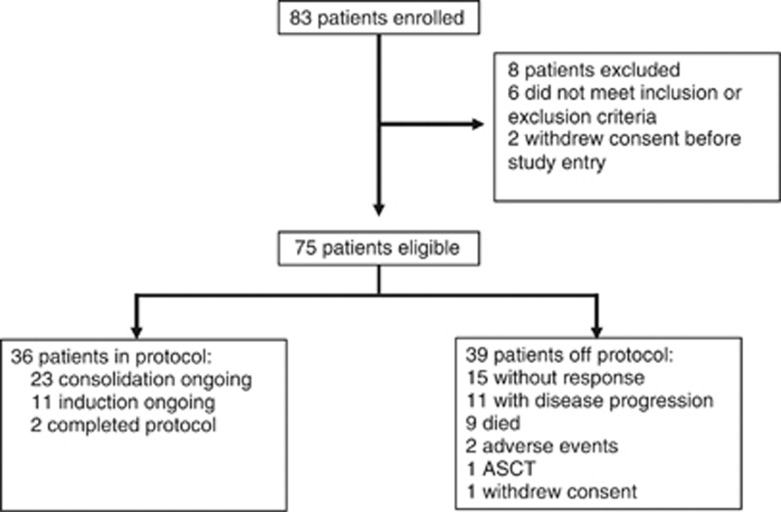
From March 2011 to June 2012, 83 patients were screened and 75 were enrolled (Figure 1). Five patients died during the first cycle before laboratory assessments were performed and 70 were evaluable for ORR. Toxicity and survival parameters were assessed in the intention-to-treat cohort (75 patients). Patients received a median of five induction cycles (range 1–6; sum 346) and three consolidation cycles (range 1–6; sum 58).
Figure 1.
Patient disposition.
Patient characteristics
Median age was 68 years (range 41–85; 42.5% >70 years), and 17.5% of patients had an Eastern Cooperative Oncology Group PS of 2. Also, 19% of patients had immunoglobulin A MM, 26.5% had stage 3 disease (by International Staging System (ISS) for MM criteria), 29% had lactate dehydrogenase (LDH) elevations and 9% had renal insufficiency (Table 1). In total, 8/36 evaluable patients (22%) had adverse cytogenetics including translocations t(4;14) and t(14;16), and/or deletion of chromosome 17. Median time from diagnosis to study enrolment was 40 months (range 4–130 months).
Table 1. Patient characteristics.
| Characteristics | n=75 |
|---|---|
| Median age, years (range) | 68 (41–85) |
| Age >70 years, n (%) | 32 (42.5) |
| Male, n (%) | 34 (45) |
| WHO performance status, n (%) | |
| 0–1 | 62 (82.5) |
| ⩾2 | 13 (17.5) |
| Myeloma type, n (%) | |
| IgG | 46 (61.5) |
| IgA | 15 (20) |
| Light chain | 14 (18.5) |
| ISS stage, n (%) | |
| I–II | 55 (73.5) |
| III | 20 (26.5) |
| FISH analysis, n (%) | |
| Standard risk | 28 (78) |
| High risk | 8 (22) |
| Renal failure, n (%) | 7 (9) |
| LDH (U/l), n (%) | |
| Normal | 53 (71) |
| Above normal range | 22 (29) |
| Hb level (g/dl), n (%) | |
| <10 | 19 (28) |
| ⩾10 | 49 (72) |
| Platelet count at enrolment ( × 109/l), n (%) | |
| <100 | 6 (8) |
| ⩾100 | 62 (92) |
| Previous lines of therapy, n (%) | |
| 1 | 40 (53) |
| 2 | 22 (29) |
| 3 | 3 (8) |
| 4 | 7 (10) |
| Prior treatment regimens | |
| Previous alkylating, n (%) | 52 (69) |
| Previous anthracyclines, n (%) | 22 (29) |
| Previous thalidomide, n (%) | 43 (57) |
| Previous bortezomib, n (%) | 35 (46.5) |
| Previous lenalidomide, n (%) | 41 (54.5) |
| Duration of lenalidomide treatment, median (range) | 10 (2–42) |
| Previous lenalidomide and bortezomib, n (%) | 15 (20) |
| Refractory to IMIDs, n (%) | 24 (32) |
| Previous ASCT, n (%) | 33 (44) |
| Disease history longer than 3 years, n (%) | 44 (59) |
Abbreviations: ASCT, autologous stem cell transplant; FISH, fluorescence in situ hybridization; Hb, haemoglobin; Ig A, immunoglobulin A; Ig G, immunoglobulin G; IMID, Immunomodulatory drug; ISS, International Staging System for Multiple Myeloma criteria; LDH, lactate dehydrogenase; WHO, World Health Organization.
All patients had received prior treatments, including thalidomide (57%), lenalidomide (54.5%) or bortezomib (46.5%). Patients had received a median of one prior therapy (range 1–4), including alkylators (69%), anthracyclines (29%) and ASCT (44%). Twenty-four patients (32%) were refractory to IMIDs (Table 1).
Response. Primary end point and impact of baseline variables
The response rate ⩾PR after four BVD cycles was 71.5% (95% CI: 66–77%), including 11 CRs (16%), 13 VGPRs (18.5%) and 26 PRs (37%). Also, 14 patients (20%) had disease stabilization. Best ORR assessed during treatment was 77% (Table 2). Median time-to-response was 1.2 months (range 0.9–1.4 months) and 78% of patients had responded at 2 months.
Table 2. Response to BVD therapy (n=70 evaluable).
| Response | After four cycles, n (%) | Best response, n (%) |
|---|---|---|
| CR | 11 (16) | 14 (20) |
| VGPR | 13 (18.5) | 14 (20) |
| PR | 26 (37) | 26 (37) |
| SD | 14 (20) | 14 (20) |
| PD | 6 (8.5) | 2 (3) |
| At least PR | 50 (71.5) | 54 (77) |
Abbreviations: BVD, bendamustine plus bortezomib and dexamethasone; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
According to univariate analysis, only prior treatment with bortezomib significantly reduced the response rate ⩾PR (54.5% vs 86.5% P=0.003; Table 3). Prior bortezomib treatment was also associated with a lower CR rate (P=0.064). Although renally insufficient patients had a lower ORR than those with normal renal function (50% vs 73.5%), sample numbers were low and the difference was not statistically significant.
Table 3. Univariate analysis of factors associated with ORR and median TTP.
| ORR (%) | P-value | Median TTP (months) | P-value | |
|---|---|---|---|---|
| Age (years) | ||||
| >70 | 76.5 | 0.401 | 16 | 0.803 |
| ⩽70 | 67.5 | 16.5 | ||
| WHO performance status | ||||
| ⩾2 | 58 | 0.270 | 15.5 | 0.401 |
| 0–1 | 74 | NR | ||
| FISH analysis | ||||
| Poor risk | 75 | 0.954 | 10.5 | 0.047 |
| Standard risk | 76 | NR | ||
| ISS stage | ||||
| III | 68.5 | 0.643 | 16.5 | 0.559 |
| I–II | 74 | NR | ||
| Renal failure | ||||
| Yes | 50 | 0.224 | 4.5 | 0.002 |
| No | 73.5 | 17 | ||
| LDH level | ||||
| Elevated | 66.5 | 0.564 | 15.5 | 0.089 |
| Normal | 73.5 | NR | ||
| Platelet count at enrolment | ||||
| <100 × 109/l | 80 | 0.606 | 10 | 0.065 |
| ⩾100 × 109/l | 70 | 16.5 | ||
| Number of previous treatments | ||||
| >2 | 61.5 | 0.382 | 9.5 | 0.002 |
| ⩽2 | 73.5 | 17 | ||
| Previous anthracyclines | ||||
| Yes | 72.5 | 0.871 | 12.5 | 0.057 |
| No | 71 | 16.5 | ||
| Previous alkylator | ||||
| Yes | 73 | 0.604 | 17 | 0.991 |
| No | 66.5 | 16 | ||
| Previous IMIDs | ||||
| Yes | 86 | 0.740 | 17 | 0.594 |
| No | 73 | NR | ||
| Previous lenalidomide | ||||
| Yes | 71 | 0.940 | 16 | 0.059 |
| No | 72 | NR | ||
| Refractory to lenalidomide | ||||
| Yes | 84 | 0.652 | 12 | 0.465 |
| No | 72 | 15.5 | ||
| Previous bortezomib | ||||
| Yes | 54.5 | 0.003 | 11.5 | 0.011 |
| No | 86.5 | NR | ||
| Previous lenalidomide and bortezomib | ||||
| Yes | 53 | 0.037 | 9 | <0.001 |
| No | 76.5 | 17 | ||
| Previous ASCT | ||||
| Yes | 63 | 0.129 | 17 | 0.737 |
| No | 79 | 16.5 | ||
| Response after four BVD cycles | ||||
| <PR | NA | NA | 11 | 0.024 |
| ⩾PR | NA | NR | ||
Abbreviations: ASCT, autologous stem cell transplant; BVD, bendamustine plus bortezomib and dexamethasone; FISH, fluorescence in situ hybridization; IMID, immunomodulatory drug; ISS, International Staging System for Multiple Myeloma criteria; LDH, lactate dehydrogenase; NA, not applicable (no data); NR, not yet reached; ORR, overall response rate; PR, partial response; TTP, time to progression; WHO, World Health Organization.
Secondary efficacy end points
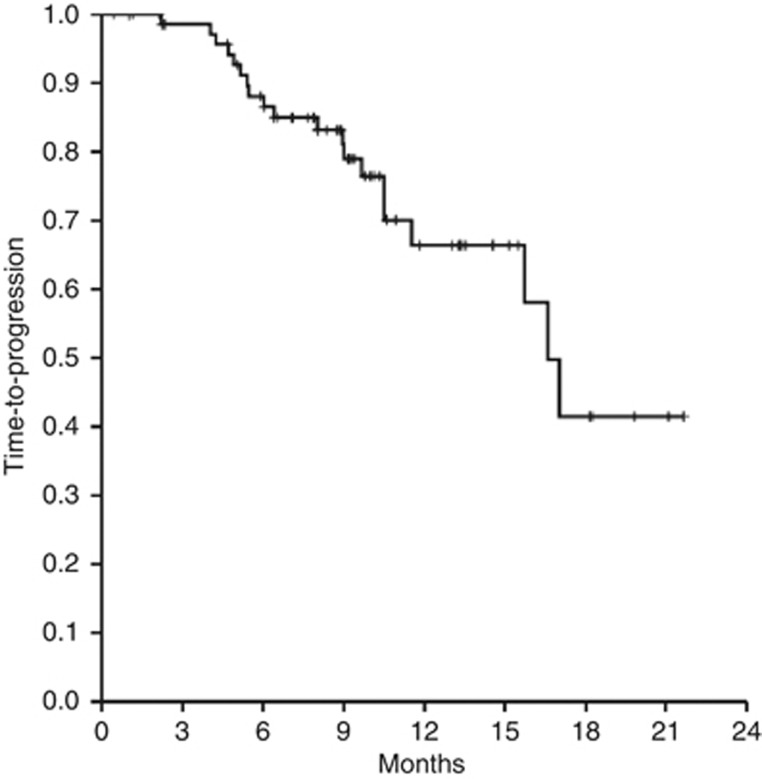
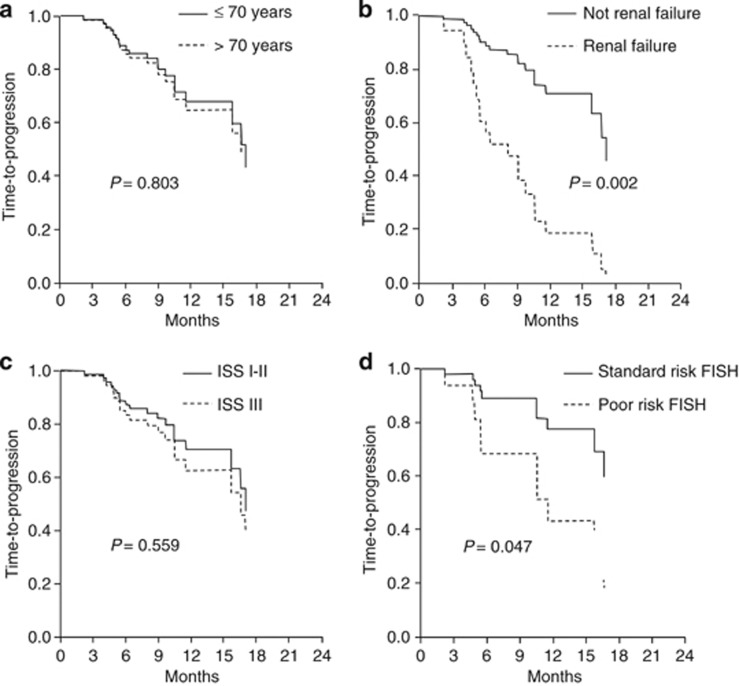
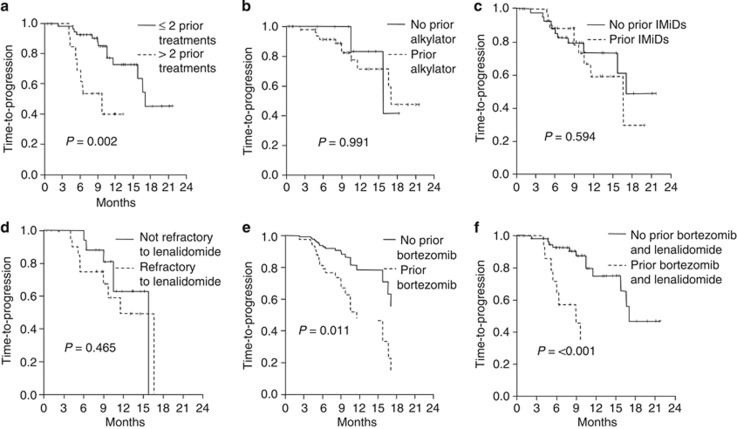
At a median follow-up of 12 months (range 6–24), 30 patients had progressed and 18 had died. Median TTP was 16.5 months (95% CI: 14.8–18.3) and 66% at 1 year (Figure 2). Median PFS was 15.5 months (95% CI: 9.5–22) and 57% at 1 year, whereas median OS had not been reached and 78% of patients were alive at 1 year. According to univariate analyses, factors that negatively affected TTP included response <PR (P=0.024), LDH above the normal range (P=0.089), more than two lines of prior therapy (P=0.002), prior therapy with anthracyclines (P=0.057), prior bortezomib (P=0.011), prior lenalidomide (P=0.059) and prior bortezomib plus lenalidomide (P<0.001). Other factors negatively correlating with TTP included renal insufficiency (TTP=4.5 months; P=0.002), thrombocytopenia (TTP=10 months; P=0.065) and adverse fluorescence in situ hybridization (FISH) (TTP=10 months; P=0.047). In contrast, patients achieving a CR had a longer TTP compared with those who did not (NR vs 14 months; P=0.073). As these latter factors were present in a minority of patients, they were excluded from the survival analysis. Cox regression analysis identified prior therapy with bortezomib plus lenalidomide as the only factor that significantly reduced TTP (9 vs 17 months; HR=4.5; 95% CI=1.7–12.3; P=0.005) (Figures 3 and 4). Regarding OS, there was no significant difference between patients aged ⩽70 or >70 years (1-year OS: 80 vs 78% P=0.918).
Figure 2.
TTP in patients treated with BVD.
Figure 3.
TTP (a) according to age, (b) to renal function (c) to disease stage (International Staging System) and (d) cytogenetics (by FISH).
Figure 4.
TTP (a) according to the number of previous therapies, (b) previous treatment with alkylating, (c) previous treatment with IMIDs, (d) refractoriness to lenalidomide, (e) previous treatment with bortezomib and (f) previous treatment with bortezomib and lenalidomide.
Safety
Fifty-five per cent and 20% of patients experienced grade 3–4 and grade 4 treatment-related adverse events (AEs), respectively. Severe AEs occurred in 12 patients (16%: eight infections, one heart failure, one sudden death, one cerebral haemorrhage and one diarrhoea with dehydration). Five patients (6.5%) experienced early death.
AEs led to therapy reduction in 15 patients (20%) mainly for peripheral neuropathy (eight patients) and thrombocytopenia (seven patients) and led to protocol discontinuation in eight patients (10.5%) due to thrombocytopenia (three patients), infections (three patients), neuropathy (one patient) and heart failure (one patient) (Table 4). The cumulative dose intensity was, however, 83 and 82% of bortezomib and bendamustine, respectively.
Table 4. AEs occurring in the 75 patients receiving BVD.
| AEs | Grade 1–2, n (%) | Grade 3–4, n (%) | Grade 5, n (%) |
|---|---|---|---|
| Anaemia | 24 (32) | 9 (12) | |
| Neutropenia | 16 (21) | 14 (18.5) | |
| Thrombocytopenia | 19 (25) | 23 (30.5) | |
| Infections | 8 (10.5) | 6 (8) | 3 (4) |
| Peripheral neuropathy | 18 (24) | 6 (8) | |
| Cardiac toxicity | 3 (4) | 1 (1) | 2 (2.5) |
| Liver toxicity | 2 (2.5) | 1 (1) | |
| Gastrointestinal toxicity | 9 (12) | 4 (5) | |
| Fatigue | 17 (23) | 2 (2.5) | |
| DVT | 2 (2.5) | 2 (2.5) |
Abbreviations: BVD, bendamustine plus bortezomib and dexamethasone; DVT, deep vein thrombosis.
The most frequent severe AEs were thrombocytopenia (30.5%), neutropenia (18.5%), infections (12% 4% grade 5: one case of septic shock and two cases of pneumonia), neuropathy (8%) and gastrointestinal and cardiovascular events (both 6.5%). The grade 2–4 peripheral neuropathy rate was similar between groups of patients with or without a history of prior bortezomib (21% vs 33% P=0.469) or thalidomide (28 vs 26% P=0.725) treatment.
Patients aged >70 years had a significantly higher incidence of grade 3–4 thrombocytopenia (22 vs 37% P=0.042) and severe infections (7 vs 19% P=0.047), and consequently a higher rate of dose reductions (9 vs 34.5% P=0.007) and discontinuations (7 vs 15.5% P=0.043) by comparison to younger patients. However, the cumulative dose intensity of bendamustine was not significantly lower in older patients compared with younger ones (76 vs 84% P=0.389).
Moreover, four of five early deaths occurred in patients aged >70 years. Other baseline patient characteristics and lines of prior therapy had no effect on compliance or toxicity in this study.
Discussion
The results of this phase II trial demonstrated that the BVD combination elicited a rapid and high response rate (>70%) of good quality (more than a third CR+VGPR) in patients with rrMM previously treated with targeted agents. Moreover, the BVD regimen was associated with median TTP and PFS durations of 16.5 and 15.5 months, respectively, and a 1-year OS of 78% in the intention-to-treat cohort.
The main outcomes of this study appear similar, and in some regard, better than results reported with the main approved salvage therapy combinations for this setting, such as bortezomib–dexamethasone (VD)10 and lenalidomide–dexamethasone (Rd).44, 45 The reported response rate obtained with VD and Rd did not exceed 60% and median TTP ranged from 9 to 13.5 months in patients who had mostly been treated with old drugs. Only a small proportion of patients receiving VD and Rd also received thalidomide in the VD and Rd studies, whereas our results with BVD have been achieved in a patient population fully treated with new targeted agents and who might therefore be considered to have a poorer prognosis (Table 5).
Table 5. Comparison of the present (BVD) study with relevant published trials of novel targeted agent combination regimens in rrMM cohorts.
| Parameter | V±D (RETRIEVE)48 (8 cycles) | V±D (CREST)10 (up to 8 cycles) | V±PLD12 (up to 8 cycles) | RD45 (continuous) | BVD (12 cycles over 18 months) | VTD49 (12 cycles) |
|---|---|---|---|---|---|---|
| Median age, years (range) | 67 (34–84) | 60 (30–84) | 61 (28–85) | 61 (28–85) | 68 (41–85) | 60 (29–76) |
| WHO performance status ⩾2 (%) | 16.0 | 15.0a | 0.0 | 13.0 | 17.5 | 36.0b |
| ISS stage III (%) | — | — | — | — | 26.5 | 14.0 |
| Median number of previous treatments (range) | 2 (1–7) | 3 (1–7) | – | – | 1 (1–4) | 1 |
| Previous therapy (%) | ||||||
| Thalidomide | 31.0 | 27.0 | 40.0c | 30.0 | 57.0 | 10.0 |
| Lenalidomide | 0.0 | 0.0 | 0.0 | 54.5 | 0.0 | |
| Bortezomib | 100.0 | 0.0 | 0.0 | 4.5 | 46.5 | 20.0 |
| ORR (%)d | 40.0 | 50.0 | 44.0 | 60.0 | 77.0 | 86.0 |
| CR (%) | 1.0 | 4.0e | 4.0 | 16.0 | 20.0 | 25.0 |
| Median TTP (months) | 8.4 | 11.0 | 9.3 | 11.3 | 16.5 | 19.5 |
| Grade 3–4 toxicity (%) | ||||||
| Neutropenia | 7.0 | 23.0 | 30.0 | 29.5 | 18.5 | 11.0 |
| Thrombocytopenia | 35.0 | 23.0 | 23.0 | 11.4 | 30.5 | 17.0 |
| Infection | 10.0 | 15.0 | 3.0 | 15.0 | 12.0 | 14.0 |
| Neuropathy | 9.0 | 15.0 | 3.0 | <1.0 | 8.0 | 31.0 |
| Discontinuations due to toxicity (%) | 21.0 | — | — | 20.0 | 10.5 | 28.0 |
| Early mortality (%) | 6.0 | 3.8 | 3.0 | 2.8 | 6.5 | — |
Abbreviations: B, bendamustine; CR, complete response; D, dexamethasone; ISS, International Staging System for Multiple Myeloma criteria; nCR, near complete response; ORR, overall response rate; PLD, pegylated liposomal doxorubicin; R, lenalidomide; rMM, refractory multiple myeloma; T, thalidomide; TTP, time to progression; V, bortezomib; WHO, World Health Organization.
Karnofsky status ⩽70.
Karnofsky status ⩽80.
Thalidomide+lenalidomide.
Best response (as the number of cycles of therapy was different between studies).
CR+nCR.
The response rates achieved in this phase II study are similar to those obtained in the four main published trials (i.e. ORR: 65–75%) that have evaluated bendamustine combined with bortezomib and steroids.33, 34, 35, 36 Despite differences in patient populations, median TTP/PFS of our patients previously treated with 1–2 lines of therapy (17 months, 65% at 1 year) are comparable to those reported by Ludwig et al.34 (13 months) and Rodon et al.35 (67% at 1 year). The median TTP/PFS achieved with BVD is also similar to the 8- to 9.5-month TTP/PFS duration achieved by Hrusovsky and Heidtmann33 and Ludwig et al.34 in patients with more advanced disease. Our findings with BVD and those reported in the literature suggest that the BVD triple regimen has reliable and reproducible efficacy in different patient populations. Moreover, it is interesting that the present study and that reported by Rodon et al.35 utilized a very similar schedule containing consolidation with 12 treatment courses over 18 months and resulted in comparable results, which are likely better than those obtained by Ludwig et al.34 with a shorter schedule of therapy (eight courses in 8 months).34
The response rate of >80% (including >20% CR rate) and median TTP (17 months) with BVD in the subgroup analyses of the present study were particularly satisfactory in patients pretreated with thalidomide. Most reported studies with VD or Rd combinations in rrMM have demonstrated a detrimental effect of prior thalidomide treatment on the response rate and median TTP duration,46, 47, 48 which was not evident with BVD in our study. This observation suggests that bendamustine may be able to overcome the hypothetical resistance induced by pretreatment with thalidomide. Moreover, the efficacy of BVD in patients previously treated with standard- or high-dose alkylating agents in our study was similar to that of the entire study population, indirectly suggesting that bendamustine has no cross-resistance with other alkylators. Similar results were reported with a triple therapy VTD (ORR=86% TTP=19 months) in a recent published study.49 Nevertheless, all patients included in this trial were in first relapse after transplant and less than a quarter of them were previously treated with new drugs. None had received prior lenalidomide (Table 5). Therefore, although better outcomes were expected with triplet therapy, the comparison among studies should be viewed with caution because of differences in patient populations, particularly regarding prior treatment that appears the most important prognostic factor for response and survival. In this regard, notably, prior treatment with lenalidomide, lenalidomide plus thalidomide and refractoriness to lenalidomide had a negligible impact on outcomes achieved by patients treated with BVD combination in our study. This finding is in contrast to results in other similar published studies,34, 35 in which pre-exposure to IMIDs was significantly associated with a lower response rate and shorter TTP. Unfortunately, there are no data regarding the number of patients receiving lenalidomide and the duration of treatment they received in the published studies, so it is not possible to compare them with our present results and draw conclusions. However, from Table 1 it is clear that a substantial proportion of patients in our trial had received lenalidomide for a reasonable duration. According to the literature, the outcome of patients receiving salvage therapies and who had been treated frontline with lenalidomide as maintenance50, 51 or as induction (i.e., melphalan, prednisone and lenalidomide) and maintenance (MPR plus lenalidomide)52 are not significantly different from that of patients not exposed to lenalidomide, suggesting that lenalidomide probably does not induce resistant relapse.
Retreatment with the same new drug/regimen represents a suitable choice when prior outcomes have been satisfactory. Recently, Petrucci et al.48 demonstrated an ORR of 40% and a median TTP of 8.4 months in patients retreated with VD. In our study, patients exposed to bortezomib had a significantly lower response rate and shorter TTP compared with those without prior exposure. Despite this, retreatment with BVD in our study still resulted in a response rate of 55% and median TTP of 11 months (i.e., longer than reported in published studies with VD), suggesting that bendamustine augmented the efficacy of VD.
Consistent with the findings of Ludwig et al.,34 patients pretreated with bortezomib and lenalidomide, and with more than two lines of therapy in our study had the worst outcomes. However, a response rate of 50–60% and median TTP of 9–10 months with BVD in these subgroups could not be defined as a dismal outlook in this difficult-to-treat patient population.
Several trials are evaluating novel second-generation targeted agents, either alone or in combination with dexamethasone, in rrMM patients with some promising preliminary results.53 Owing to its distinct mechanism of action, and efficacy and acceptable tolerability in rrMM patients who had received prior targeted agents in our study, bendamustine would be a logical choice for further evaluation in combination with novel second-generation targeted agents in clinical trials in this setting.
In our study, the efficacy of BVD was unaffected by disease characteristics, such as age, PS, ISS stage or elevated LDH, suggesting that this regimen is independent of the most important unfavourable prognostic factors in rrMM. Somewhat surprisingly, the six patients with renal insufficiency exhibited the worst response rate and quality of response (50% ORR, 0% CR) and the poorest TTP (median 4.5 months). Although renal insufficiency is a well-known and important adverse prognostic factor in MM,54 better results were expected in this subgroup of patients with BVD. According to Pönisch et al.,36 a small number of patients, receiving bendamustine plus prednisone and bortezomib, who had thrombocytopenia had a poorer outcome compared with patients without thrombocytopenia. Similarly, in our study, median TTP was shorter with BVD in patients with thrombocytopenia, although it was still a non-trivial 10 months in duration. The median TTP was also shorter with BVD in eight patients with adverse cytogenetics by comparison to patients with standard risk FISH analysis in our study. The median TTP with BVD was also a non-trivial 10 months in this small subgroup at adverse risk. However, because of the small number of patients in each subgroup, it is not possible to draw clear conclusions on the effectiveness of BVD.
Depth of response to BVD may have an impact on outcomes as suggested by the trend towards prolonged median TTP in patients achieving a CR vs other responses.
The BVD regimen was generally well tolerated in our study in patients of median age 68 years, with treatment reductions and discontinuations in 20 and 10% of patients, respectively. These rates are similar to, or lower than, those reported with VD (22–35% reductions and 21–23% discontinuations)10, 48 and Rd (20% discontinuations).44, 45 The proportion of patients who discontinued BVD therapy in our study was similar to that reported by Rodon et al.35 (15 vs 11%), using the same schedule in patients older than 65 years. However, it is worth noting that in older patients, the BVD dose reduction/interruption rate is two- to threefold higher than that in younger patients. In our study, the discontinuation rate was twofold higher in patients >70 years compared with younger patients. Despite this, the cumulative dose intensity of BVD in older patients was similar to that in younger patients. This may explain the lack of difference in terms of response and survival parameters between the two groups of patients. Mortality rate (6.5%), mainly due to infections, was of concern in our protocol as it was two times the rate reported in trials evaluating dual therapies (Table 5). Nevertheless, all deaths due to infection occurred in the first 30 patients before antibacterial prophylaxis was recommended. Furthermore, nearly all early deaths occurred in the elderly subgroup of patients (4 of 5 deaths in patients >70 years). Despite being a triple therapy, BVD was associated with similar mortality in our study to bortezomib as monotherapy or dual therapy (±dexamethasone) in a retreatment study reported by Petrucci et al.48 (i.e., 6.5 vs 6%). Median age was similar in the Petrucci study and our study but definitely higher than that in the dual therapy studies (Table 5). From these data we can argue that mortality depends mostly on age and supportive care rather than on the number of drugs administered. In any case, although BVD had acceptable tolerability in the whole study population of median age 68 years, caution is warranted when considering this triple regimen for patients older than 70 years, in which an adapted dose BVD regimen should be considered. Dose-adapted therapy in older patients, regardless of the regimen, is one of the most important recent skill acquisitions in MM treatment.55 The tolerability findings of our study therefore provide valuable information that should facilitate adequate management of BVD in future studies and in clinical practice.
The incidence of thrombocytopenia in our study and the Rodon et al.35 study was lower than that reported in the studies of Ludwig (35%) et al.34 and Pönisch (42%) et al.36 As the dose of bendamustine was very similar in the four studies, it could be argued that delaying the second dose of bendamustine (i.e., administering on day 8 as in our study rather than days 2 or 4) may reduce thrombocytopenia. The lowest rate of thrombocytopenia (10%) was seen in the Rodon et al. study,35 in which bortezomib was administered weekly to patients in first relapse. On the contrary, the highest thrombocytopenia documented in the Pönisch et al. study36 may be related to a 21-day instead of a 28-day treatment cycle, despite the lower dose of bendamustine (60 mg/m2) administered. However, the rate of thrombocytopenia in our study (30%) was similar or lower than that reported with VD (30–35%)10, 48 but higher than that reported with Rd (17%).44, 45 In contrast, the rate of severe neutropenia with BVD in our study appears lower than that reported with Rd (18 vs 36%).44, 45
Infections (mainly pneumonia and sepsis) were the main non-haematologic complication with BVD in our study. However, the BVD infection rate (15–20%) is quite similar to that reported with Rd (15–16%)44, 45, 56 and VD (15%).10, 48 Adequate antibiotic prophylaxis should therefore be administered in the first cycles of BVD to reduce this potentially dangerous complication.
Neuropathy is one of the most important side effects with bortezomib therapy.57 However, in our study, neuropathy was rarely found to be a factor limiting BVD feasibility. Neuropathy was also not a limiting factor in patients previously treated with bortezomib or thalidomide according to similar published studies,33, 34, 35, 36 whereas it was a concern in patients treated with VTD,48 in which the incidence was indubitably higher (31% Table 5). Weekly and subcutaneous administration of bortezomib could further reduce the occurrence of troublesome neuropathy.58, 59
In conclusion, the BVD combination is a feasible and effective regimen in patients with rrMM. This triple regimen appears to be more effective than approved dual regimens, without added toxicities in patients pretreated with IMIDs and with a median of one prior line of therapy. Notably, BVD appears to retain its efficacy in advanced rrMM disease. Caution is warranted in older patients in whom adequate dose adaptation and sound infection prophylaxis are advisable. According to these phase II results, BVD warrants further evaluation in phase III studies in comparison with approved salvage regimens for rrMM.
Acknowledgments
We acknowledge Dr Laurence De-Netto, Wells HealthCare Communications Limited, for providing editorial assistance that was funded by Mundipharma International who had no input into the analysis and interpretation of the study data. We thank Mundipharma for providing bendamustine and all data managers, including Rak Patel, for their excellent collaboration. This study was funded by a grant provided by Mundipharma.
The authors have previously received honoraria from Mundipharma, Janssen, Celgene, Novartis, Amgen and Sanofi.
References
- Jakubowiak A. Management strategies for relapsed/refractory multiple myeloma. Semin Hematol. 2012;49 (Suppl 1:S16–S32. doi: 10.1053/j.seminhematol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H, Avet-Loiseau H, Bladé J, Boccadoro M, Cavenagh J, Cavo M, et al. European perspective on multiple myeloma treatment strategies: update following recent congresses. Oncologist. 2012;17:592–606. doi: 10.1634/theoncologist.2011-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment' approaches in the era of novel agents. Leukemia. 2012;26:73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, San-Miguel JF, Anderson KC. Emerging therapies for the treatment of relapsed or refractory multiple myeloma. Eur J Haematol. 2011;86:1–15. doi: 10.1111/j.1600-0609.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- Mikhael JR, Belch AR, Prince HM, Lucio MN, Maiolino A, Corso A, et al. A high response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br J Haematol. 2009;144:169–175. doi: 10.1111/j.1365-2141.2008.07409.x. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Nagler A, Sonneveld P, Bladé J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- Terpos E, Kastritis E, Roussou M, Heath D, Christoulas D, Anagnostopoulos N, et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22:2247–2256. doi: 10.1038/leu.2008.235. [DOI] [PubMed] [Google Scholar]
- Kim YK, Sohn SK, Lee JH, Yang DH, Moon JH, Ahn JS, et al. Clinical efficacy of a bortezomib, cyclophosphamide, thalidomide, and dexamethasone (Vel-CTD) regimen in patients with relapsed or refractory multiple myeloma: a phase II study. Ann Hematol. 2010;89:475–482. doi: 10.1007/s00277-009-0856-x. [DOI] [PubMed] [Google Scholar]
- Lee SS, Suh C, Kim BS, Chung J, Joo YD, Ryoo HM, et al. Bortezomib, doxorubicin, and dexamethasone combination therapy followed by thalidomide and dexamethasone consolidation as a salvage treatment for relapsed or refractory multiple myeloma: analysis of efficacy and safety. Ann Hematol. 2010;89:905–912. doi: 10.1007/s00277-010-0943-z. [DOI] [PubMed] [Google Scholar]
- Offidani M, Corvatta L, Polloni C, Gentili S, Mele A, Rizzi R, et al. Thalidomide, dexamethasone, Doxil and Velcade (ThaDD-V) followed by consolidation/maintenance therapy in patients with relapsed-refractory multiple myeloma. Ann Hematol. 2011;90:1449–1456. doi: 10.1007/s00277-011-1217-0. [DOI] [PubMed] [Google Scholar]
- Montero LFC, Solano F, Gomez-Roncero MI, Calle C, Cano I, Foncillas MA, et al. Bortezomib in combination with high-dose dexamethasone for relapsed multiple myeloma. Haematologica. 2006;91 (Suppl 1:abstract 1210. [Google Scholar]
- Morris TCM, Drake M, Kettle P, Hamilton J, Burnside P, Kyle A, et al. Use of bortezomib in northern Ireland–combination with dexamethasone routinely used to improve response rate. Haematologica. 2007;92 (Suppl 2:abstract P-637. [Google Scholar]
- Harrison SJ, Quach H, Dean J, Milner A, Copeman MC, Prince HM, et al. Bortezomib and dexamethasone from cycle 1 as treatment and maintenance for multiple myeloma relapse (The BoMeR trial): impact on response and time to progression. J Clin Oncol. 2010;28 (Suppl:abstract 8151. [Google Scholar]
- Dimopoulos MA, De Samblanx HM, Roussou MG, Zervas K, Katodritou E, Sargin D, et al. Efficacy of bortezomib plus dexamethasone versus bortezomib monotherapy in patients with relapsed/refractory multiple myeloma: an interim report from an International electronic observational study Blood 2010116abstract 3027. [Google Scholar]
- Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–317. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- Leoni LM, Hartley JA. Mechanism of action: the unique pattern of bendamustine-induced cytotoxicity. Semin Hematol. 2011;48 (Suppl 1:S12–S23. doi: 10.1053/j.seminhematol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Gaul L, Mandl-Weber S, Baumann P, Emmerich B, Schmidmaier R. Bendamustine induces G2 cell cycle arrest and apoptosis in myeloma cells: the role of ATM-Chk2-Cdc25A and ATM-p53-p21-pathways. J Cancer Res Clin Oncol. 2008;134:245–253. doi: 10.1007/s00432-007-0278-x. [DOI] [PubMed] [Google Scholar]
- Strumberg D, Harstrick A, Doll K, Hoffmann B, Seeber S. Bendamustine hydrochloride activity against doxorubicin-resistant human breast carcinoma cell lines. Anticancer Drugs. 1996;7:415–421. doi: 10.1097/00001813-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Balfour JAB, Goa KL. Bendamustine. Drugs. 2001;61:631–638. doi: 10.2165/00003495-200161050-00009. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, Vermorken JB. Phase I studies with bendamustine: an update. Semin Oncol. 2002;29 (Suppl 13:15–18. doi: 10.1053/sonc.2002.34874. [DOI] [PubMed] [Google Scholar]
- Michael M, Bruns I, Boolke E, Zohren F, Czibere A, Safaian NN, et al. Bendamustine in patients with relapsed or refractory multiple myeloma. Eur J Med Res. 2010;15:13–19. doi: 10.1186/2047-783X-15-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damay G, Malard F, Hulin C, Caillot D, Garidi D, Royer B, et al. Efficacy of bendamustine unrelapsed/refractory myeloma patients: results from the French compassionate use program. Leuk Lymphoma. 2012;53:632–634. doi: 10.3109/10428194.2011.622422. [DOI] [PubMed] [Google Scholar]
- Aguado Bueno B, Andres IV, Entrena L, De Arriba F, Krisnik I, Navas B, et al. Preliminary experience of the spanish compassionate use registry of bendamustine in patients with relapsed and/or refractory multiple myeloma Blood 2012120abstract 4035. [Google Scholar]
- Pönisch W, Rozanski M, Goldschmidt H, Hoffmann FA, Boldt T, Schwarzer A, et al. Combined bendamustine, prednisolone and thalidomide for refractory or relapsed multiple myeloma after autologous stem-cell transplantation or conventional chemotherapy: results of a phase I clinical trial. Br J Haematol. 2008;143:191–200. doi: 10.1111/j.1365-2141.2008.07076.x. [DOI] [PubMed] [Google Scholar]
- Grey-Davies E, Bosworth JL, Boyd KD, Ebdon C, Saso R, Chitnavis D, et al. Bendamustine, thalidomide and dexamethasone is an effective salvage regimen for advanced stage multiple myeloma. Br J Haematol. 2011;156:545–555. doi: 10.1111/j.1365-2141.2011.08887.x. [DOI] [PubMed] [Google Scholar]
- Fenk R, Michael M, Zohren F, Graef T, Czibere A, Bruns I, et al. Escalation therapy with bortezomib, dexamethasone and bendamustine for patients with relapsed or refractory multiple myeloma. Leuk Lymphoma. 2007;48:2345–2351. doi: 10.1080/10428190701694194. [DOI] [PubMed] [Google Scholar]
- Hrusovsky I, Heidtmann HH.Combination therapy of bortezomib with bendamustine in elderly patients with advanced multiple myeloma. Clinical observation Blood 2007110abstract 4851. [Google Scholar]
- Ludwig H, Kasparu H, Greil R, Leitgeb C, Muldur E, Rauch E, et al. Treatment with bendamustine–bortezomib–dexamethasone (BBD) in relapsed/refractory multiple myeloma shows significant activity and is well tolerated Blood 2012120abstract 943. [Google Scholar]
- Rodon P, Hulin C, Pegourie B, Tiab M, Anglaret B, Benboubker L, et al. Bendamustine, bortezomib and dexamethasone (BVD) in elderly patients with multiple myeloma in first relapse: updated results of the Intergroupe Francophone Du Myelome (IFM) 2009–01 Trial Blood 2012120abstract 4044. [Google Scholar]
- Pönisch W, Bourgeois M, Moll B, Heyn S, Jäkel N, Wagner I, et al. Combined bendamustine, prednisone and bortezomib (BPV) in patients with relapsed or refractory multiple myeloma. J Cancer Res Clin Oncol. 2013;139:499–508. doi: 10.1007/s00432-012-1339-3. [DOI] [PubMed] [Google Scholar]
- Berenson JR, Yellin O, Bessudo A, Boccia RV, Noga SJ, Gravenor DS, et al. Phase I/II trial assessing bendamustine plus bortezomib combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013;160:321–330. doi: 10.1111/bjh.12129. [DOI] [PubMed] [Google Scholar]
- Lentzsch S, O'Sullivan A, Kennedy RC, Abbas M, Dai L, Lalo Pregja S, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119:4608–4613. doi: 10.1182/blood-2011-12-395715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SK, Krishnan A, Roy V, Zimmermann TM, Gertz MA, Stockerl-Goldstein KE, et al. Phase I/II, multicenter, open-label, dose-escalation study of bendamustine in combination with lenalidomide and dexamethasone (BRD) in patients with relapsed multiple myeloma: a Multiple Myeloma Research Consortium Study Blood 2012120abstract 2965. [Google Scholar]
- Pönisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone—a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO) Cancer Res Clin Oncol. 2006;132:205–212. doi: 10.1007/s00432-005-0074-4. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang X, Chen L, Liang J, Suvannasankha A, Abonour R, et al. Synergistic activity of bendamustine in combination with doxorubicin and bortezomib in multiple myeloma cells Blood 2008112abstract 5171. [Google Scholar]
- Durie BGM, Harousseau JL, Miguel JS, Barlogie B, Anderson K, Gertz M, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- Wang M, Dimopoulos MA, Chen C, Cibeira MT, Attal M, Spencer A, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Soulier J, Fermand J-P, Yakoub-Agha I, Attal M, Hulin C, et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leukemia. 2010;24:623–628. doi: 10.1038/leu.2009.273. [DOI] [PubMed] [Google Scholar]
- Petrucci MT, Giraldo P, Corradini P, Teixeira A, Dimopoulos MA, Blau IW, et al. A prospective, international phase 2 study of bortezomib retreatment in patients with relapsed multiple myeloma. Br J Haematol. 2013;160:649–659. doi: 10.1111/bjh.12198. [DOI] [PubMed] [Google Scholar]
- Garderet L, Iacobelli S, Moreau P, Dib M, Lafon I, Niederwieser D, et al. Superiority of the triple combination of bortezomib–thalidomide–dexamethasone over the dual combination of thalidomide–dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 randomized phase III trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2475–2482. doi: 10.1200/JCO.2011.37.4918. [DOI] [PubMed] [Google Scholar]
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos M, Petrucci MT, Foa R, Catalano JV, Kropff M, Yu Z, et al. Analysis of second-line lenalidomide following initial relapse in the MM-015 Trial Blood 2012120abstract 944. [Google Scholar]
- Moreau P. The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. Semin Hematol. 2012;49 (Suppl 1:S33–S46. doi: 10.1053/j.seminhematol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–9226. doi: 10.1200/JCO.2005.03.2086. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Bladé J, Mateos MV, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN) Blood. 2011;118:4519–4529. doi: 10.1182/blood-2011-06-358812. [DOI] [PubMed] [Google Scholar]
- Caravita T, Offidani M, Siniscalchi A, Gentili S, Caraffa P, Perrotti A, et al. Infection complications in an unselected cohort of patients with multiple myeloma treated with lenalidomide combinations. Eur J Haematol. 2012;89:276–277. doi: 10.1111/j.1600-0609.2012.01814.x. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Delforge M, Beksac M, Wen P, Jongen JL, Sezer O, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608. doi: 10.1038/leu.2011.346. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, et al. Bortezomib–melphalan–prednisone–thalidomide followed by maintenance with bortezomib–thalidomide compared with bortezomib–melphalan–prednisone for initial treatment of MM. J Clin Oncol. 2010;28:5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study Lancet Oncol 201112431–440.801. [DOI] [PubMed] [Google Scholar]