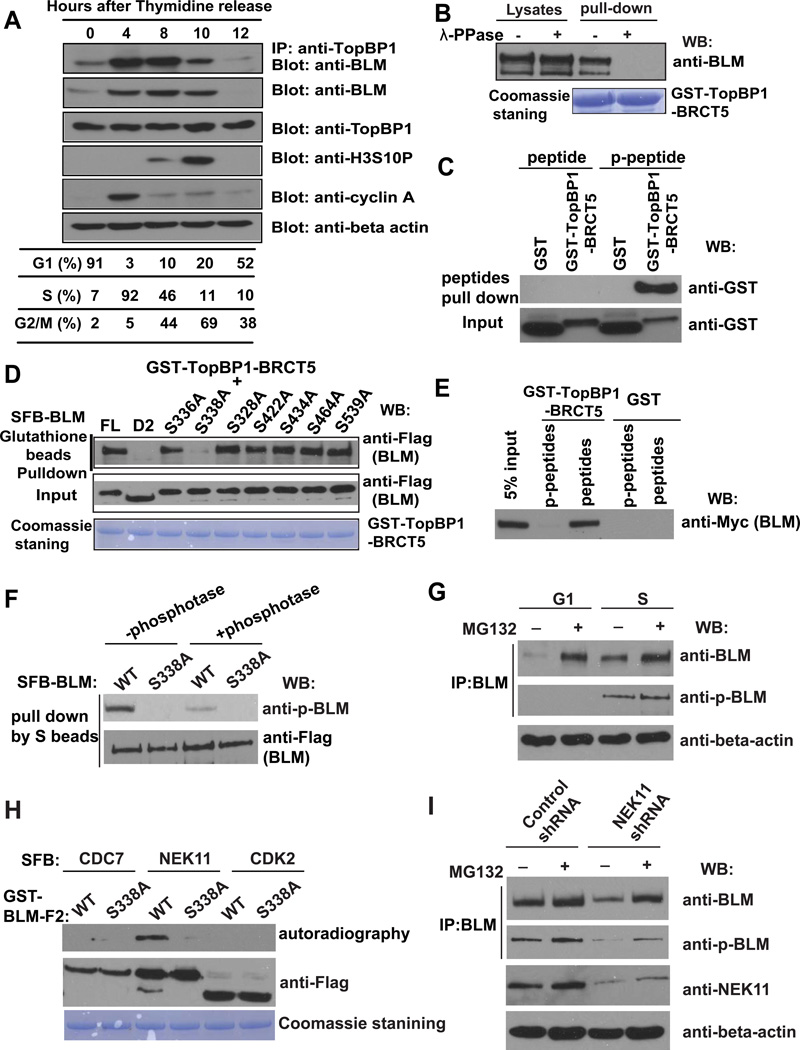
Figure 2. TopBP1/BLM Interaction Is Cell Cycle–Regulated.
(A) The TopBP1/BLM interaction is cell cycle–regulated. HeLa cells were synchronized by double thymidine block, and then released in fresh medium without thymidine and collected at the indicated time points. Cell lysates were prepared, immunoprecipitation and immunoblotting experiments were performed using antibodies as indicated. Cell cycle distributions were confirmed by fluorescence-activated cell sorting analysis and summarized in the table.
(B) TopBP1/BLM interaction is phosphorylation dependent. Beads coated with bacterially expressed GST-TopBP1 BRCT5 fusion protein were incubated with cell lysates containing exogenously expressed, SFB-tagged BLM that was mock treated or treated with λ lambda protein phosphatase. Immunoblotting experiments were carried out with the indicated antibodies.
(C) Mapping phosphorylation site on BLM that is important for its interaction with TopBP1. S336A, S338A, S328A, S422A, S434A, S464A, and S539A are mutants in which a known phosphorylated serine site was changed to alanine. Beads coated with bacterially expressed GST-TopBP1 BRCT5 fusion protein were incubated with cell lysates containing exogenously expressed, SFB-tagged wild-type or mutant BLM. Immunoblotting experiments were carried out using indicated antibodies.
(D) Phosphorylated or control BLM peptides were incubated with purified GST or GST-BRCT5 fusion proteins. Input (lower panel) and GST-fusion proteins associated with peptides (upper panel) were assessed by immunoblotting using anti-GST antibodies.
(E) Beads coated with bacterially expressed GST or GST-BRCT5 fusion proteins were incubated with cell lysates containing exogenously expressed, Myc-tagged BLM together with phosphorylated or control BLM peptides. Immunoblotting experiments were carried out using indicated antibodies.
(F) Cell lysates containing SFB-tagged wild-type or S338A mutant of BLM were treated with or without λ-phosphotase and subjected to immunoprecipitation using S protein beads. The immunoprecipitates were immunoblotted with indicated antibodies.
(G) HeLa cells were synchronized in G1 phase or S phase. Cell lysates were prepared, and immunoprecipitated with BLM antibody. Western blotting was performed using antibodies as indicated.
(H) SFB-CDC7, SFB-NEK11 and SFB-CDK2 were purified from 293T cells by S protein beads and incubated with eluted GST-BLM-F2 (aa 250–480) and γ-33P-ATP for 60 minutes in kinase buffer. The supernatant were subjected for autoradiography and the beads were subjected to Western blotting with anti-Flag antibody.
(I) HeLa cells were infected with NEK11 shRNA or control shRNA. Cell lysates were subjected to immunoprecipitation using anti-BLM antibody. The immunoprecipitates were blotted with indicated antibodies.