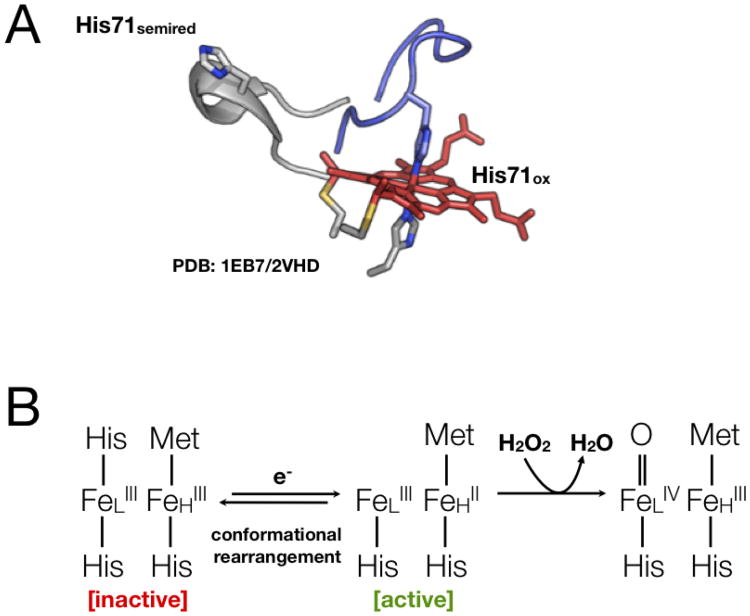
Fig. 1.
A. The conformational switch found in the majority of known bCcP enzymes involves the reorganization of the distal face of the peroxidatic heme, as a function of the redox state of a high-potential heme, some 12 Å away. B. The mechanistic impact of reductive activation suggests that by “banking” an electron in the high potential center, the first kinetic intermediate need to involve the build-up of a radical species.