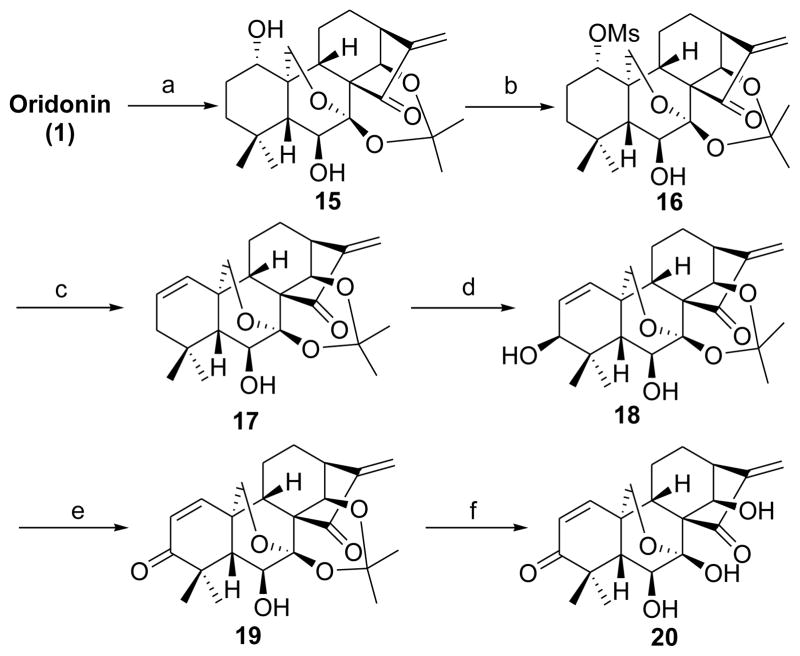
Scheme 3. Synthesis of the dienone analogues 19 and 20a.
aReagents and conditions: a) MeCH(OMe)2, cat. p-TsOH, acetone, rt, 2 h, 93%; b) MsCl, Et3N, CH2Cl2, rt, overnight, 80%; c) LiBr, Li2CO3, DMF, 110 °C, 2 h, 84%; d) SeO2, 1,4-dioxane, 110 °C, 16 h, 76%; e) PDC, CH2Cl2, rt, 4 h, 80%; f) 5% HCl (aq), MeOH, CH2Cl2, rt, 1 h, 85%.