Table 1.
Inhibition of HIV-1 RNase H enzymatic activity by thienopyrimidinones carrying C2 aromatic substitutions. IC50 values represent the average of triplicate analysis.
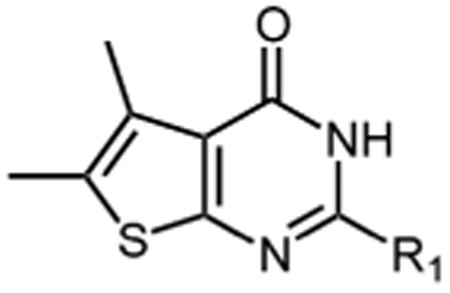
| |||||
|---|---|---|---|---|---|
|
| |||||
| Compound | R1 | IC50 (μM) | Compound | R1 | IC50 (μM) |
| mono-substituted phenyl | di-substituted phenyl | ||||
| 1 |
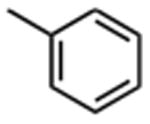
|
11.3 ±0.6 | 9 |
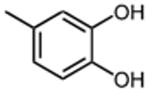
|
0.26 ±0.01 |
| 2 |
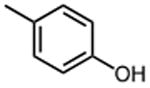
|
0.79 ±0.11 | 10 |
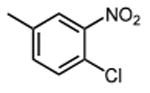
|
12.0 ±0.6 |
| 3 |
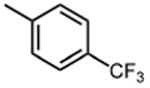
|
1.3 ±0.4 | 11 |
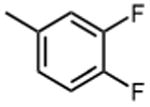
|
>50 |
| 4 |
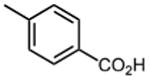
|
1.6 ±0.4 | 12 |
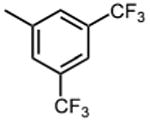
|
>50 |
| 5 |
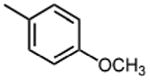
|
9.1 ±2.5 | 13 |
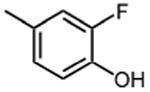
|
>50 |
| 6 |
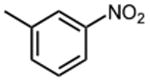
|
1.5 ±0.1 | 14 |
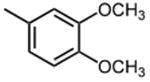
|
12.6 ±0.8 |
| 7 |
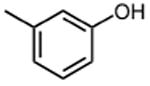
|
>50 | 15 |
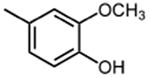
|
>50 |
| 8 |
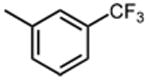
|
>50 | |||
