Abstract
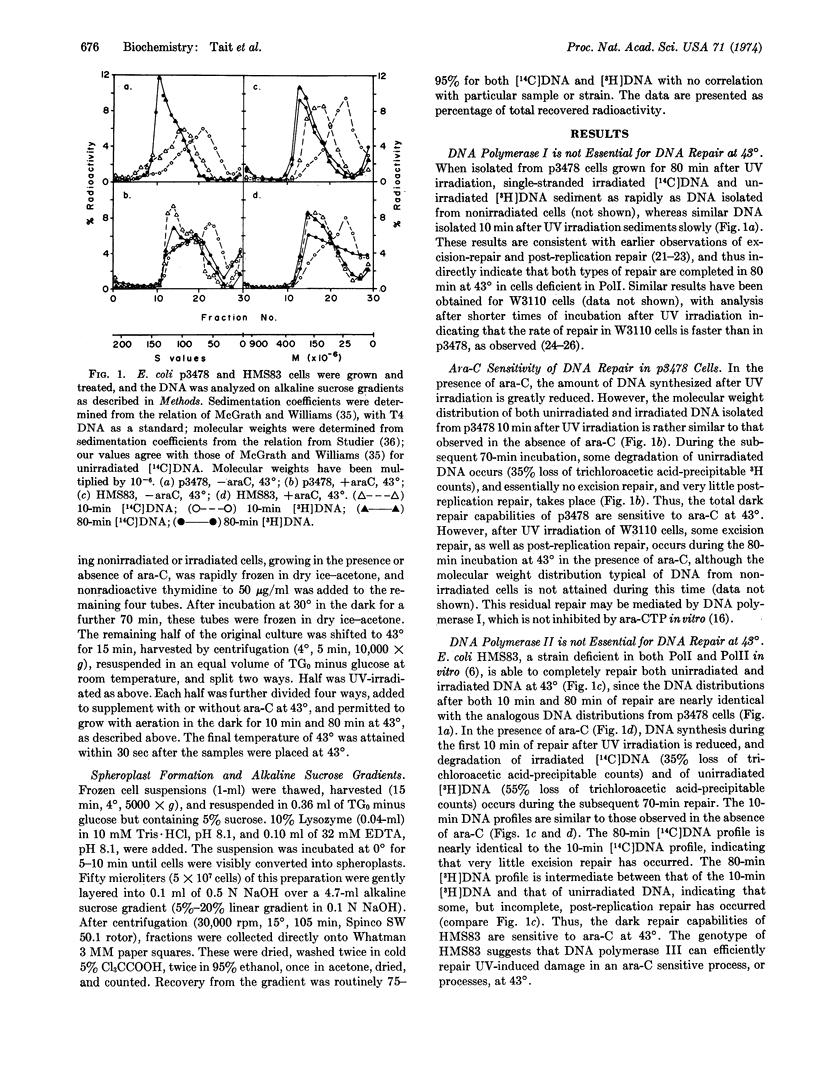
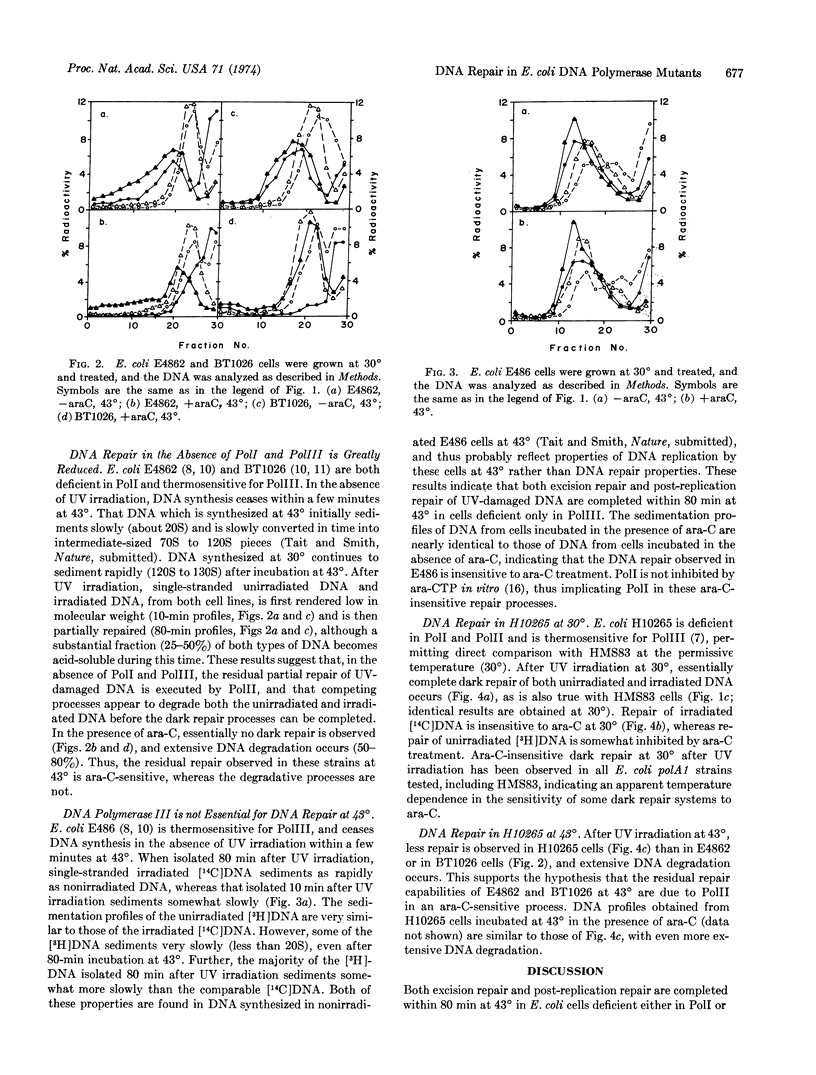
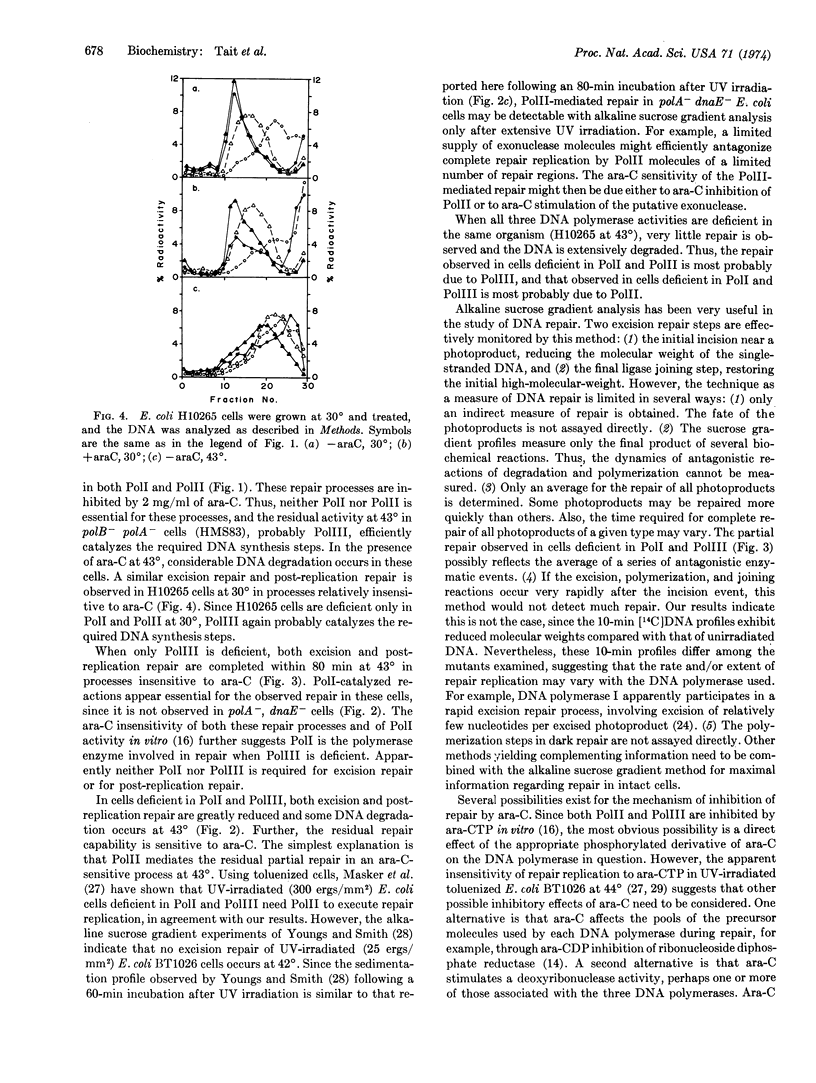
E. coli mutants deficient in DNA polymerase I, in DNA polymerases I and II, or in DNA polymerase III, can efficiently and completely execute excision repair and post-replication repair of UV-damaged DNA at 43° when assayed by alkaline sucrose gradients. Repair by cells deficient in polymerase I and in polymerases I and II is inhibited by 1-β-D-arabinofuranosylcytosine at 43°, whereas that by cells deficient in polymerase III is insensitive to the inhibitor. When both DNA polymerases I and III are deficient, both excision repair and post-replication repair are greatly reduced at 43°, and the residual repair capability is inhibited by 1-β-D-arabinofuranosylcytosine. Very little dark repair is observed in cells deficient in DNA polymerases I, II, and III, and the DNA is extensively degraded. These results suggest that either DNA polymerase I or DNA polymerase III is required for complete and efficient repair, and that when both DNA polymerases I and III are deficient, DNA polymerase II mediates a limited, incomplete dark repair of UV-damaged DNA. DNA polymerases I and III thus appear to be important enzymes in both DNA replication and DNA dark repair.
Keywords: polA1, polB, dnaE mutants; UV irradiation; 1,β-D-arabinofuranosylcytosine; alkaline sucrose gradients
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Harris N., Karon M. Kinetics of 1-beta-D-arabinofuranosylcytosine-induced chromosome breaks. Cancer Res. 1970 Oct;30(10):2477–2483. [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S. Introduction to the biochemistry of D-arabinosyl nucleosides. Prog Nucleic Acid Res Mol Biol. 1966;5:1–88. doi: 10.1016/s0079-6603(08)60231-7. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Role of DNA polymerase I and the rec system in excision-repair in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1156–1160. doi: 10.1073/pnas.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Possible involvement of DNA polymerase I in excision of RNA from Col E1 DNA in vivo. Nat New Biol. 1973 Sep 12;245(141):39–41. doi: 10.1038/newbio245039a0. [DOI] [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Gefter M., Mindich L. A mutant of Escherichia coli defective in DNA polymerase II activity. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3238–3242. doi: 10.1073/pnas.69.11.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. DNA synthesis in cell-free extracts of a DNA polymerase-defective mutant. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1348–1355. doi: 10.1016/0006-291x(70)90014-8. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. Purification and DNA synthesis in cell-free extracts: properties of DNA polymerase II. Proc Natl Acad Sci U S A. 1971 Apr;68(4):761–764. doi: 10.1073/pnas.68.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel P. L., Veomett G. E. A possible function of DNA polymerase in chromosome replication. Biochem Biophys Res Commun. 1970 Nov 25;41(4):973–980. doi: 10.1016/0006-291x(70)90180-4. [DOI] [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Ultraviolet-stimulated DNA synthesis in toluenzied Escherichia coli deficient in DNA polymerase I. Proc Natl Acad Sci U S A. 1973 Jan;70(1):129–133. doi: 10.1073/pnas.70.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W., Hanawalt P., Shizuya H. Role of DNA polymerase II in repair replication in Escherichia coli. Nat New Biol. 1973 Aug 22;244(138):242–243. doi: 10.1038/newbio244242a0. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. A new DNA polymerase activity of Escherichia coli. I. Purification and properties of the activity present in E. coli polA1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1557–1564. doi: 10.1016/0006-291x(70)90565-6. [DOI] [PubMed] [Google Scholar]
- Nüsslein V., Otto B., Bonhoeffer F., Schaller H. Function of DNA polymerase 3 in DNA replication. Nat New Biol. 1971 Dec 29;234(52):285–286. doi: 10.1038/newbio234285a0. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Arisawa M., Sugino A. Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2954–2957. doi: 10.1073/pnas.68.12.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama Reddy G. V., Goulian M., Hendler S. S. Inhibition of E. coli DNA polymerase II by ara-CTP. Nat New Biol. 1971 Dec 29;234(52):286–288. doi: 10.1038/newbio234286a0. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Repair replication of DNA in ultraviolet irradiated Mycoplasma laidlawii B. J Mol Biol. 1969 Nov 28;46(1):57–72. doi: 10.1016/0022-2836(69)90057-6. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Schaller H. E., Bonhoeffer F. J. DNA synthesis in vitro. Nature. 1970 May 23;226(5247):711–713. doi: 10.1038/226711a0. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Ginsberg B., Berkower I., Hurwitz J. Deoxyribonucleic acid plymerase II. of Escherichia coli. I. The purification and characterization of the enzyme. J Biol Chem. 1972 Jan 25;247(2):489–497. [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Involvement of DNA polymerase 3 in excision repair after ultraviolet irradiation. Nat New Biol. 1973 Aug 22;244(138):240–241. doi: 10.1038/newbio244240a0. [DOI] [PubMed] [Google Scholar]



