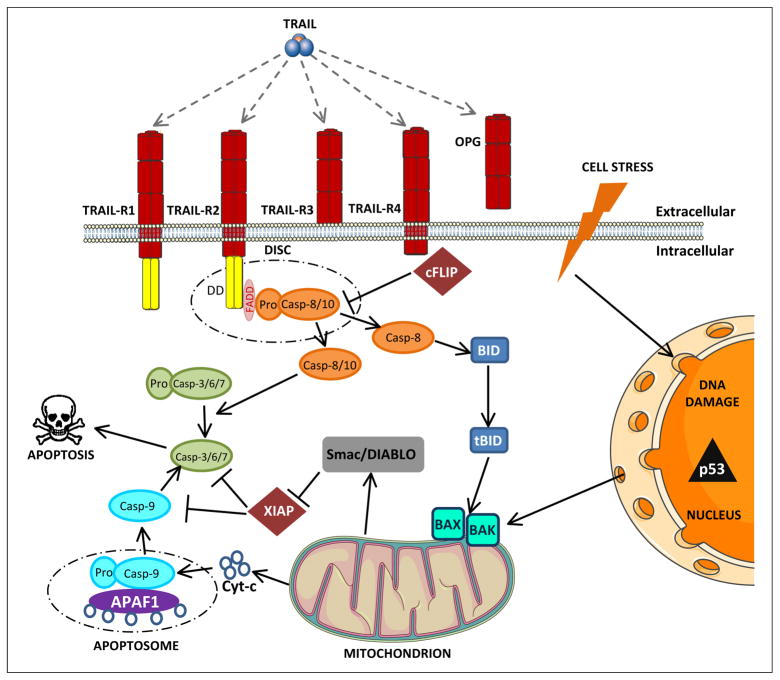
Figure 2. Apoptosis overview.
Apoptosis occurs through two pathways. The extrinsic apoptosis pathway occurs independently of p53 and requires the binding of pro-apoptotic ligands such as TRAIL. TRAIL can bind to four membrane-bound receptors (TRAIL-R1-4) and one soluble receptor (OPG). TRAIL-R1 and TRAIL-R2 contain a cytoplasmic death domain (DD) through which TRAIL can transmit an apoptotic signal. TRAIL-R3 and TRAIL-R4 also bind TRAIL, but because of the absence of a DD they are unable to initiate signaling. For this reason they are called decoy receptors. Binding of TRAIL to TRAIL-R1/2 leads to recruitment of the adaptor FADD and initiator procaspase-8 and 10 to rapidly form the DISC. Procaspase-8 and 10 are cleaved to form caspase-8 and 10 which activate effector caspase-3, 6 and 7 committing the cell to apoptose. At the DISC, variants of a protease-deficient caspase homolog called cFLIP inhibit the activation of caspase-8 and thus the propagation of apoptotic signaling. The intrinsic apoptosis pathway is initiated in response to cell stress such as chemotherapy and radiotherapy. This results in DNA damage causing the p53 oncogene to activate the pro-apoptotic Bcl-2 family proteins BAK and BAX. Pro-apoptotic proteins cytochrome c and Smac/DIABLO are released from mitochondria. Cytochrome c forms a protein complex, the apoptosome, with APAF-1 and activates caspase-9 which in turn activates effector caspase-3, 6 and 7 resulting in apoptosis. Smac/DIABLO inhibits apoptosis-inhibiting proteins such as XIAP, thus amplifying apoptotic signaling. APAF-1, Apoptotic protease activating factor-1; Bcl-2, B cell chronic lymphocytic leukaemia/lymphoma 2; BAK, Bcl-2 homologous antagonist/killer; BAX, Bcl-2-associated protein; FADD, Fas-associated death domain; cFLIP, cellular FLICE- inhibitory protein; DISC, Death-inducing signaling complex; SMAC, second mitochondria-derived activator of caspase; XIAP, X-linked inhibitor of apoptosis protein.