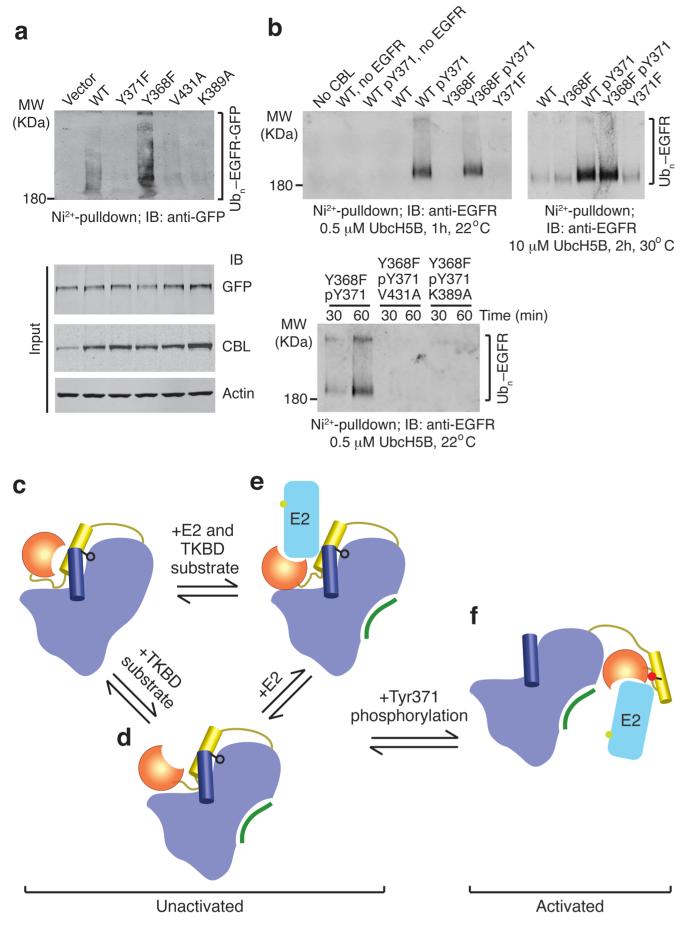
Figure 5.
EGFR ubiquitination by CBL. (a) Immunoblots from in vivo EGFR ubiquitination by full-length CBL variants. Ni2+-pulldown products were Western blotted for GFP to detect His-Ub-EGFR-GFP. Protein input levels were assessed with the indicated antibodies. (b) Western blots from in vitro EGFR ubiquitination by CBL47–435 variants. Ni2+-pulldown products were Western blotted for EGFR to detect His-Ub-EGFR. (c–f) Model for autoinhibition and phosphorylation-dependent activation of c-Cbl. c-Cbl exists in an unactivated state (c–e) where its E2-binding affinity is reduced by a competitive RING autoinhibitory mechanism. Coloring of c-Cbl, E2 and substrate is as in Fig. 1. (c) In the absence of E2, c-Cbl adopts a closed conformation where the RING’s E2-binding surface associates with the TKBD. (d) TKBD substrate binding induces partial RING opening. (e) E2 binding causes the RING domain to adopt an open conformation. The TKBD competes against E2 for RING binding, reducing E2 affinity and E3 activity. In the unactivated state, Tyr371 (black ball-and-stick) secures the LH to the TKBD and limits the RING domain rotation to a region distal from the TKBD substrate-binding site. (f) pTyr371 (red ball-and-stick) activates c-Cbl by releasing LH from the TKBD, thereby abolishing autoinhibition, altering LH-RING-E2 interactions and promoting dramatic LHR conformational changes that bring the RING domain and E2 into proximity of substrate.