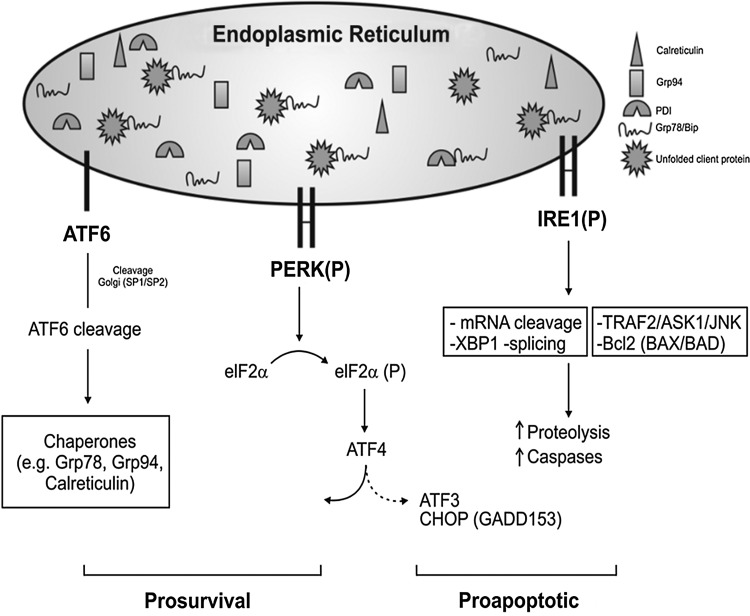
FIG. 3.
A depiction of the unfolded protein response (UPR) signaling pathways. Inositol-requiring protein-1 kinase (Ire1) is the most ancient of all the UPR signaling conserved from yeast to mammalian. It works as an endoribonuclease, slicing the mRNA of the transcription factor X-box binding protein-1 (XBP1). Ire1 is closely linked to apoptosis, activating the c-Jun amino-terminal kinase (JNK) and also caspase-12 via interaction with TRAF2 and with proapoptotic Bcl-2-associated proteins (BAX/BAK), and it can also promote apoptosis via apoptosis signal regulating kinase 1 (ASK1)/JNK signaling. ATF6 is processed by SP1/2 proteins in the Golgi generating cleavage ATF6 that promotes ER stress-related chaperones. PERK: protein kinase RNA (PKR)-like ER kinase (PERK) autophosphorylation promotes the phosphorylation of the α subunit of the downstream eukaryotic initiation factor2 (eIF2α-P) to strongly inhibit mRNA translation and shuts down global protein synthesis (9, 25, 40, 54, 93). In a paradoxical way, this phosphorylation allows the synthesis of certain stress proteins such as the ATF4 transcriptional factor via an open reading frame (ORF)-dependent translation mechanism. Other kinases that promote eIF2α phosphorylation include heme-regulated inhibitor kinase, general control non-derepressible 2 kinase, PKR, and, thus, eIF2α-P is known as a part of the Integrated Stress Response. The ATF4, XBP1, and ATF6 regulate genes encoding ER chaperones, ERAD, and a broad range of metabolic process ranging from aminoacid transport to proteins and phospholipid synthesis and adaptive response. ATF4 along with the ATF6 activation is more associated to survival outcome in response to ER stress. In contrast, increased levels of CHOP (or Gadd153) expression along with IRE1 activation are related to more pro-apoptotic events in response to ER stress.