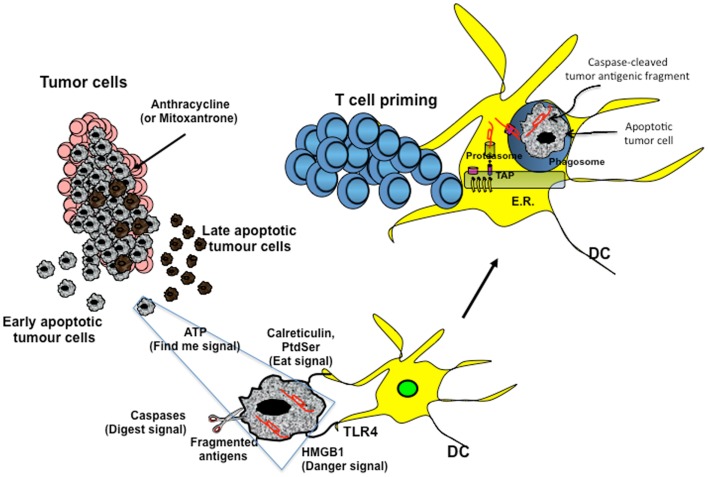
Figure 1.
Cross-presentation of chemotherapy-associated antigens derived from apoptotic tumor cells leading to anti-tumor immunity. Tumor cells, upon chemotherapy treatment (i.e., anthracycline), undergo immunogenic cell death. According to the Kroemer–Zitvogel model, the immunogenic death (apoptosis, necrosis, autophagy, etc.) of cancer cells involves a multistep process, including the release of “find-me” signals (such as fractalkine, nucleotides, and ATP) that attract phagocytes or dendritic cells (DCs), the expression of “eat-me” signals [such as phosphatidylserine (PtdSer) and calreticulin] that facilitate recognition by phagocytes or DCs, and, finally, the release of danger-associated molecular patterns [such as high mobility group box 1 protein (HMGB1) and other signals described in the text] that enable dying tumor cells to lose the propensity to induce tolerance and to stimulate powerful anticancer immune responses. An additional factor that is involved in the success of the immunogenic chemotherapy may emerge from the capacity of caspases to cut and release apoptotic cell-associated antigenic fragments (in red in the figure), thus facilitating their transport from phagosomes into the cytosol and the processing by DCs (“digest-me” signals) via the class I-processing pathways [the figure emphasizes the model suggesting that caspase-cleaved apoptotic fragments are trimmed by cytosolic proteasomes in the form of peptides and that TAPs transport the resulting apoptotic self epitopes into the lumen of the endoplasmic reticulum (ER), where they can bind the appropiate class I molecule]. The final goal of these multiple checkpoints is to cross-present tumor epitopes and to elicit a wide repertoire of memory tumor-specific CD8+ T cells in patients undergoing tumor regression in response to appropriate chemotherapeutic regimes. These tumor-specific T cells represent a principal tool for discovering immunogenic tumor antigens by interrogating those responding to highly purified tumor proteins.