Table 1.
Hoy's solubility parameters of the tested monomers. They represent index of the intermolecular forces that occurs between molecules. In particular, δd is the dispersive contribution (i.e. all non-polar contribution), δp the polar contribution, δh the hydrogen bond contribution and δt represents the total solubility parameter.
| Abbreviations | Chemical name | Molecular Structure | δd | δp | δh | δt |
|---|---|---|---|---|---|---|
| TEGDMA | Triethylene glycol dimethacrylate |
|
14.2 | 10.1 | 8.2 | 19.2 |
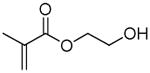
| HEMA | 2-hydroxyethyl methacrylate |
|
13.3 | 12.3 | 15.2 | 23.6 |
| UDMA | Urethane dimethacrylate |
|
17.0 | 12.4 | 2.8 | 21.3 |
| 2-MP | Bis[2-(methacryloyloxy)ethyl] phosphate |
|
14.9 | 14.8 | 14.3 | 25.4 |
| TCDM | Di(hydroxyethyl methacrylate)ester of 5-(2,5,-dioxotetrahydrofurfuryl)-3-methyl-3-cyclohexenyl-1,2-dicarboxylic anhydride |

|
18.7 | 15.1 | 7.4 | 25.1 |
| Bis-GMA | 2,2-bis[4-(2-hydroxy-3-methacryloyloxy propoxy)]-phenyl propane |
|
16.6 | 13.4 | 5.8 | 22.1 |
