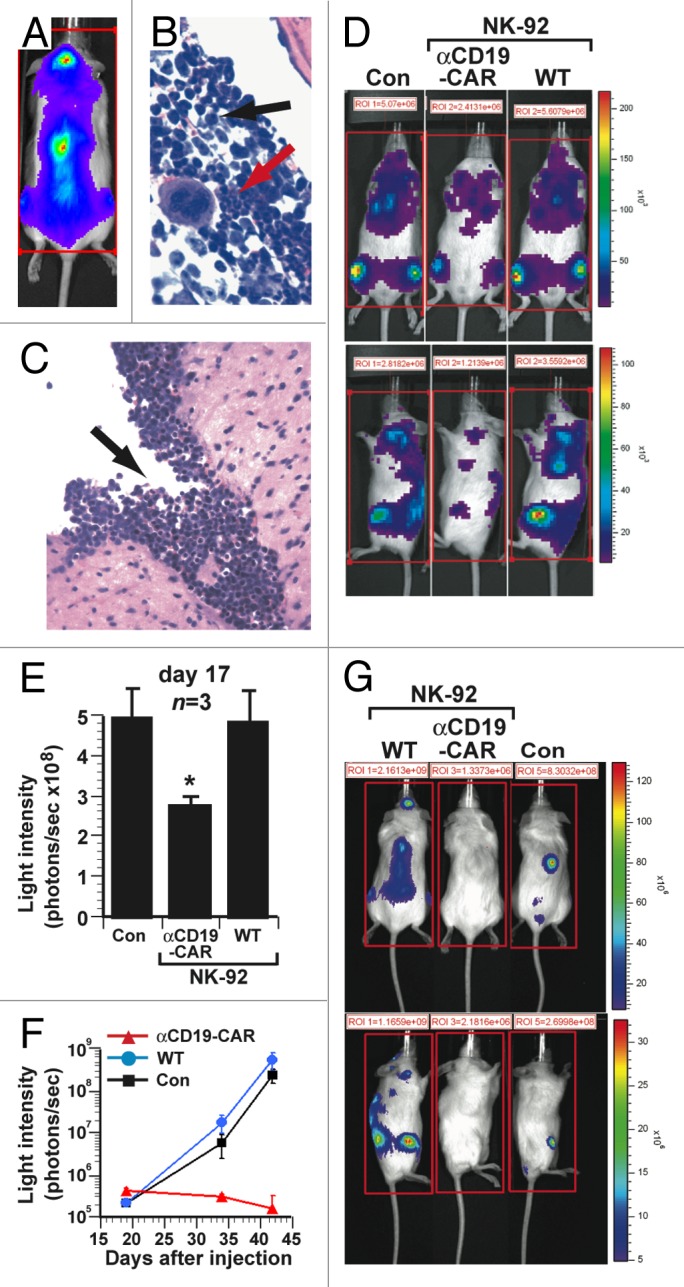
Figure 2. Therapeutic efficacy of NK-92 cells expressing CD19-specific CAR in a xenograft model of aggressive human B-cell acute lymphoblastic leukemia. (A–C) Immunocompromised NOD scid gamma (NSG) mice were inoculated i.v. with 1 × 106 luciferase (Luc)-expressing SUP-B15 acute lymphoblastic leukemia (ALL) cells. (A) Bioluminescence imaging (dorsal view) of mice 17 d after the injection of ALL cells, showing localization to the spine and calvarium. (B) Infiltration of bone marrow by SUP-B15 leukemia cells (black arrow) adjacent to area of normal hematopoiesis (red arrow). (C) Leptomeningeal SUP-B15 leukemic infiltrate. (D–E) NSG mice (n = 3) were inoculated i.v. with 2 × 105 Luc-expressing SUP-B15 cells and subsequently with 1 × 107 parental NK-92 cells (WT), 1 × 107 NK-92 cells expressing CD19-targeting chimeric antigen receptors (αCD19-CAR) or PBS control (Con), on days 1 to 5 post-inoculation. (D) Bioluminescence imaging of representative mice 17 d after the inoculation of ALL cells. (E) Quantification of leukemic burden from the experiment in (D) on day 17. The total bioluminescence (average of 4 sides measurements) in mice receiving NK-92 cells expressing the CD19-targeting CAR was significantly lower than in animals treated with parental NK-92 cells (*p = 0.02). (F-G) NSG mice (n = 3) were inoculated i.v. with 1 × 103 Luc-expressing SUP-B15 cells and then treated with 1 × 107 parental NK-92 cells (WT), 1 × 107 NK-92 cells expressing CD19-targeting CAR (αCD19-CAR) or PBS control (Con), on days 4, 5, and 6. (F) Mean serial bioluminescence of cohorts of leukemic mice. (G) Bioluminescence images of representative mice from cohorts in (F) at day 42. Statistical analyses were performed by unpaired Student t-tests.
