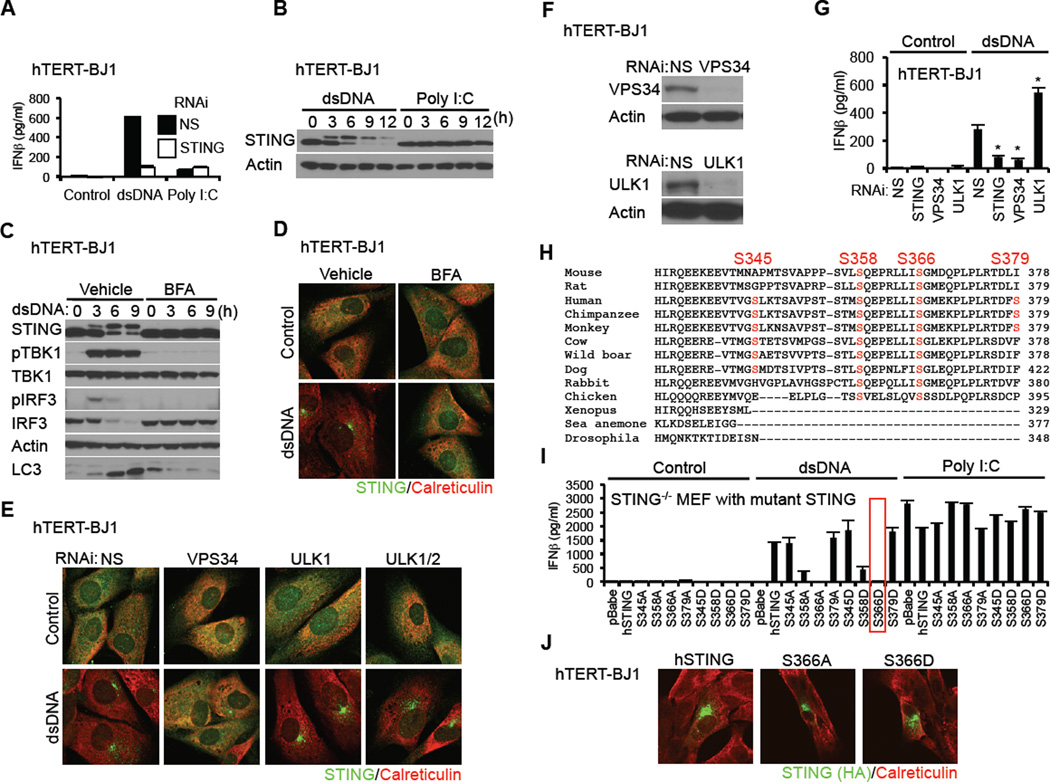
Figure 1. Phosphorylation of STING S366 Inhibits Type I IFN Production in dsDNA Signaling.
(A) hTERT-BJ1 cells were transfected with dsDNA (4 µg/ml) or poly I:C (4 µg/ml) using lipofectamine 2000 for 16 hr. IFNβ level was measured by ELISA.
(B) hTERT-BJ1 cells were transfected with dsDNA or poly I:C as described in Figure 1A for the indicated times and STING was detected by immunoblot.
(C and D) hTERT-BJ1 cells were incubated with ethanol (vehicle) or BFA (0.05 µg/ml) for 1 hr and then transfected with dsDNA (4 µg/ml) for the indicated times (C) or 6 hr (D). Immunoblot (C) or immunostaining (D) was performed with the indicated antibodies; lack of LC3 processing (bottom band) indicated loss of autophagy.
(E) hTERT-BJ1 cells were treated with siRNA (NS: Non-specific siRNA) for 3 days and then transfected with dsDNA (4 µg/ml) for 6 hr. Immunostaining was performed with the indicated antibodies.
(F) Immunoblot was performed to confirm knockdown efficiency of ULK1 and VPS34.
(G) siRNA-treated hTERT-BJ1 cells were transfected with dsDNA (4 µg/ml) for 16 hr and IFNβ was measured by ELISA.
(H) Alignment of STING amino acid sequences. Highlighted amino acids indicate dsDNA-dependent phosphorylated serines, as detected by mass spectrometry.
(I) Primary STING−/− MEF cells were reconstituted with STING variants using retroviruses. The cells were transfected with dsDNA (4 µg/ml) or poly I:C (4 µg/ml) for 16 hr and IFNβ measured by ELISA.
(J) hTERT-BJ1 cells were transfected with HA-tagged mutant hSTING for 36 hr and immuno-stained with the indicated antibodies.
Asterisks indicate significant difference (P < 0.05) compared to NS determined by Student’s t-test. Error bars indicate sd. See also Figure S1.