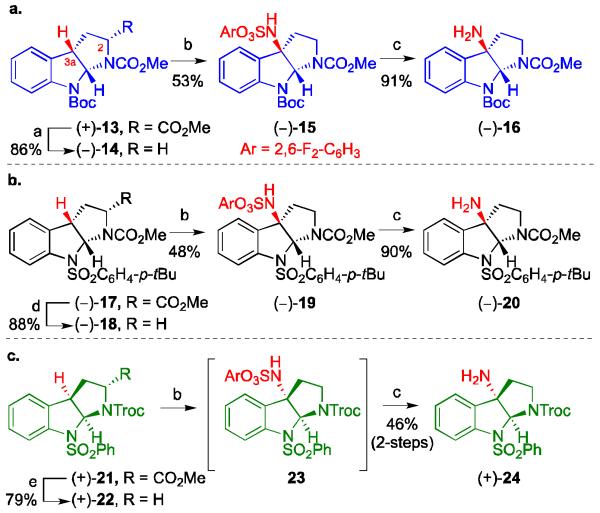
Scheme 2.
C–H Amination, synthesis of amines (−)-16, (−)-20 and (+)-24. Conditions: (a) i) 5 N KOH (aq), MeOH, 23 °C; ii) TCFH, thiopyridine N-oxide, DMAP, Et3N, THF, then t-BuSH, hν;, 23 °C, 86%; (b) Rh2(esp)2, H2NSO3Ar, PhI(OAc)2, Ph(CH3)2CCO2H, MgO, 5 Å MS, i-PrOAc, 23 °C, for 14→15, 53%, 18→19, 48%; (c) pyridine, MeCN, H2O, 70 °C, for 15→16, 91%, 19→20, 90%, 22→24, 46% (2 steps); (d) i) 5 N KOH (aq), MeOH, 23 °C; ii) (COCl)2, DMF, CH2Cl2, 23 °C; iii) (Me3Si)3SiH, AIBN, PhMe, 80 °C, 88%; (e) i) Me3SnOH, DCE, 80 °C; ii) TCFH, thiopyridine N-oxide, DMAP, Et3N, THF, then t-BuSH, hν, 23 °C (79%, 2 steps); DMF = dimethylformamide, AIBN = azobisisobutyronitrile, TCFH = N,N,N-’,N’-tetramethylchloroformamidinium hexafluorophosphate, DMAP = 4-(dimethylamino)pyridine, THF = tetrahydrofuran, DCE = 1,2-dichloroethane.