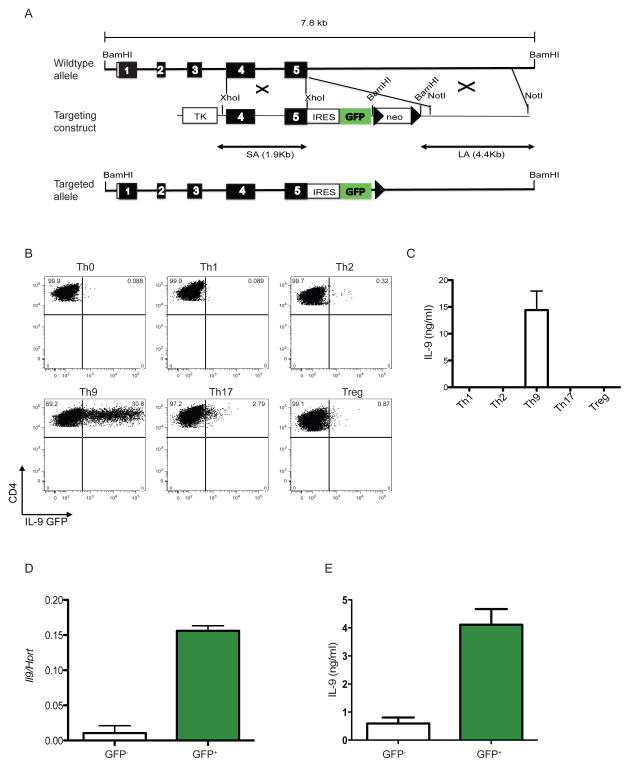
Figure 3. Generation of INFER mouse and expression analysis in vitro.
(A) A 7.8 kb genomic fragment of the Il9 locus was used to create the targeting construct (middle), in which an IRES-GFP cassette followed by a loxp-flanked neomycin resistance gene was inserted downstream of the stop codon. Deletion of neo by Cre recombinase resulted in the final targeted allele (bottom). Filled boxes represent exons 1–5. Loxp sites are shown as arrow heads. Restriction sites are depicted. TK: thymidine kinase.
(B) Sorted naïve CD4+CD62L+CD25−CD44− T cells from INFER mice were cultured for 5 days in Th0, Th1, Th2, Th9, Th17 or Treg polarizing conditions in vitro. Recovered cells were stained for surface CD4 and GFP expression was analyzed by flow cytometry. Numbers indicate the frequencies of CD4+GFP+ cells. The experiment was performed five times with similar results.
(C) Supernatants from cells isolated from cultures in (B) at day 5, stimulated in vitro with anti-CD3 for 24 hrs and analyzed by ELISA for IL-9 quantification.
(D) Sorted GFP− or GFP+ cells from day 5 Th9 cultures with INFER naïve CD4+ T cells were examined for IL-9 expression by real time RT-PCR. Mean +/− SEM normalized to Hprt is shown.
(E) GFP− or GFP+ cells as described in (D) were stimulated with anti-CD3 for 24 hrs. Supernatants where collected and analyzed by ELISA for IL-9 quantification.