Abstract
Background
Communication problems impede effective symptom management during chemotherapy. The primary aim of this pilot randomized controlled trial was to test the effects of a personal digital assistant–delivered communication intervention on pain, depression, and fatigue symptoms among breast cancer patients undergoing chemotherapy. Secondary aims included assessment of 1) study feasibility, 2) patient and clinician responses to study participation, and 3) intervention effects on health-related quality of life (HRQoL) and communication self-efficacy.
Methods
Intervention group participants (n = 27) completed symptom inventories at baseline, once per week during treatment, and at posttreatment. Depending on symptom severity, they viewed race-concordant videos on how to communicate about pain, depression and/or fatigue, using the personal digital assistant. Symptom records were tracked and shared with clinicians. Control group participants (n = 23) received usual care. Longitudinal random effects modeling assessed the changes in average symptom scores over time. Descriptive statistics assessed study feasibility and intervention effects on HRQoL and communication self-efficacy. Postintervention focus groups, interviews, and surveys assessed responses to study participation.
Results
Mean age of the participants was 51.0 years; 42 participants (84%) were white. In comparison with control, intervention group participants reported lower average pain severity over time (P = .015). Mean pain interference scores over time were marginally different between groups (P = .07); mean depression and fatigue scores over time were statistically nonsignificant. Feasibility outcomes and perspectives about study participation were positive. Mean pre–post decreases in HRQoL were generally higher among intervention group participants; pre–post changes in communication self-efficacy were equivalent.
Conclusion
Mixed findings of the study indicate the need for future research.
Cancer patients often experience multiple physical, functional, and psychosocial symptoms while undergoing chemotherapeutic treatment (1–3). Fatigue is considered one of the most problematic side effects of chemotherapy, with high and fluctuating prevalence rates (4). Levels of emotional distress can also be high, and depression may worsen during treatment (5,6). Difficulties with pain during chemotherapy are also common, particularly stomach pain and muscular aches and pains, and pain symptoms can last 1 week or more during half the cycles (7,8)
The diagnosis and treatment of cancer generates a threat to patients’ well-being, one that necessitates effective health communication (9). During cancer treatment, patient–clinician communication is important to the effective management of physical and psychological symptoms (10,11). Unfortunately, despite the high incidence of fatigue, depression, and pain in cancer patients, evidence suggests that patient–physician communication regarding these symptoms is deficient (12). Studies have documented numerous barriers that contribute to negative outcomes, such as undertreated pain and increased risk of hospitalization (13,14). Yet, improving patients’ communication skills may benefit not only individual patients but also cancer care as a whole (15).
Interactive health communication tools have proliferated in recent years, together with a growing trend toward empowering patients to take a more active role in treatment (16). Mobile devices have experienced tremendous growth in health-care settings (17,18). For instance, personal digital assistants (PDAs) have been used for symptom monitoring and education for patients receiving cancer treatment (19–21). Previous research has indicated that teaching effective communication strategies to patients may increase their participation in health care, in addition to improving adherence and health outcomes (22–24). To the authors’ knowledge, however, mobile devices have not been used for patient communication training (20,25–27).
We designed a pilot randomized controlled trial to test the effects of a personal digital assistant–delivered communication intervention on patients’ perceptions of pain, depression, and fatigue symptoms among breast cancer patients undergoing chemotherapy. Secondary aims included assessment of 1) study feasibility, 2) patient and clinician responses to participation in the study, and 3) intervention effects on measures of health-related quality of life (HRQoL) and communication self-efficacy.
Methods
Recruitment
This study was conducted from 2007 to 2009 at two outpatient cancer clinics associated with the Ohio State University Comprehensive Cancer Center. Patients were eligible to participate if they were 1) female adults aged at least 18 years, with clinical or pathological stages I–III breast cancer (any T stage and N stage) receiving treatment with adjuvant chemotherapy for the first time; 2) not pregnant; 3) able to speak and understand English; and 4) willing and able to provide written consent.
Recruitment occurred during the clinic visit before the initiation of adjuvant chemotherapy. Oncologists provided a brief introduction to the study and inquired about patients’ interest in participation. If a patient expressed interest, the research assistant then met with the patient, explained the study in detail, answered questions, and obtained written consent. Written, informed consent was obtained from each participant. The study protocol was approved by the Ohio State University Institutional Review Board. A description of the intervention is available in the online supplementary material.
Primary Measures
Pain: The Brief Pain Inventory—Short Form.
Pain was assessed using the 15-item Brief Pain Inventory—Short Form (BPI—SF). Questions assess the average level of pain and pain interference during the past week using an 11-point scale (0–10; high scores denoting greater severity and/or interference). Cronbach alpha coefficients for the scale ranged from .78 to .96 (28, 29).
Depression: The Center for Epidemiologic Studies–Depression Scale.
The Center for Epidemiologic Studies–Depression Scale (CES-D) was used to screen for depression severity. Responses are on a Likert-type scale ranging from 0 (None or rarely [less than 1 day per week]) to 3 (Most or all the time [5–7 days per week]) and include 20 common affective and somatic symptoms of depression experienced in the past week (30). Cronbach alpha coefficients ranged from .83 to .91 (30,31).
Fatigue: The Fatigue Symptom Inventory (FSI).
Fatigue was assessed with the 14-item Fatigue Symptom Inventory (FSI) (32). Questions assess the average level of fatigue and perceived interference of fatigue using an 11-point scale (0–10; high scores denoting greater severity and/or interference). Cronbach alpha for the FSI ranged from .90 to .94 (32,33).
Secondary Measures
Health-Related Quality of Life: SF-36.
The SF-36 was used to assess perception of health-related quality of life (HRQoL) during the past 4 weeks. The instrument contains 36 items and 8 individual subscales: physical functioning, physical role, bodily pain, general health, vitality, social functioning, emotional role, and mental health (34). Scores range from 0 to 100, with 100 being the more favorable score. Cronbach alpha for the SF-36 ranged from .82 to .93 (35,36).
Self-Efficacy: Communication and Attitudinal Self-Efficacy–Cancer Measure.
This 12-item instrument measures communication and attitudinal self-efficacy (CASE) in patients with cancer. It assesses three domains: 1) understanding and participating in care; 2) maintaining a positive attitude; and 3) seeking and obtaining information (37). Scores for CASE–Cancer are on a four-point scale (1–4, higher scores denoting higher self-efficacy). Cronbach alpha for CASE–Cancer ranged from .75 to .76 (37,38).
Demographic Characteristics.
Participant demographic information, including age, race, marital status, education, employment, occupation, household income, and computer use (per week), was measured at baseline. In addition, participants were asked about PDA use before the study.
At baseline and postintervention, all participants completed the BPI-SF, CES-D, FSI, SF-36, and CASE–Cancer instruments.
Patient Perspective Regarding Study Participation
To assess responses to study participation, three focus groups and five in-depth interviews were conducted with intervention group participants by two staff members (a white woman and an African American woman) of the Behavioral Measurement Shared Resource (BMSR) at the Ohio State University Comprehensive Cancer Center. Both women had experience conducting focus groups and interviews. Separate focus group sessions were organized for African American and white women. All interviews and focus groups were audio recorded and transcribed verbatim. Transcripts were reviewed for accuracy. Names of participants and other identifiers (eg, physicians’ names) were removed.
Provider Perspective Regarding Study Participation
Clinicians who participated in the study were asked to complete a 15-item postintervention survey. Providers were asked to rate intervention components (ie, inventories, videos, and graphs), the value of patients’ study participation, and assess whether they would recommend this intervention for future breast cancer patients. A five-point Likert response scale, ranging from a score of 1 (strongly disagree) to 5 (strongly agree), was used for all questions.
Analyses
Analysis of Patient-Reported Outcomes Data and Demogr aphics.
A random-effects linear regression model was used to test our hypothesis that symptom severity and interference over time would be lower for patients in the intervention group, compared with the same in the control group. Random-effects regression modeling was used to evaluate this comparison due to the longitudinal nature of the observations; this enabled us to control for within- and between-subject variability when estimating the standard errors used to test the regression coefficients. These linear regression models were adjusted for professional occupation because this demographic characteristic was not balanced during randomization. All analyses were conducted using Stata, version 9.2 (Stata Corporation, College Station, TX).
The sample size for our study was based on the largest of three sample sizes generated from the BPI-SF, CES-D, and FSI (28,30,32). All three sample sizes used the following criteria: alpha = .05 to test statistical significance, power to detect a difference in the groups at 80%, and correlation between repeated measures of 0.7. Sample size estimation was based on Frison and Pocock repeated-measure change approach (39). The FSI, CES-D, and BPI-SF produced different sample size estimates. The FSI uses a 10-point scale (0–10) to measure fatigue. The difference from the beginning to the end of the study was expected to be one unit, with an SD of 2.2 units. It produced the largest estimate (25 subjects per arm) and was used as the basis for our sample size.
Descriptive statistics were used to describe pre–post differences between intervention and control groups on the HRQoL and CASE–Cancer measures. Descriptive statistics were also used to characterize research participants’ demographics. Fisher exact methods were used to test for differences in categorical variables across groups, whereas a two-sided t test was used to test for differences in age across groups. In addition, we used random-effects linear regression where symptom was regressed on previous PDA use to assess for statistically significant differences in symptom scores.
Analysis of Postintervention Focus Group/Interview Data.
Two Behavioral Measurement Shared Resource staff members trained in qualitative analysis read through focus group/interview transcripts and generated a list of topics that categorized the perspectives and ideas shared by participants. All transcripts were coded using NVivo qualitative software and were coded independently. Codes were reviewed and discrepancies were resolved through consensus. Themes were identified based on the number of focus groups or interviews in which each topic was discussed.
Analysis of Postintervention Survey Data.
Descriptive statistics were used to summarize responses to the health-care provider survey using means and SDs.
Results
Participants
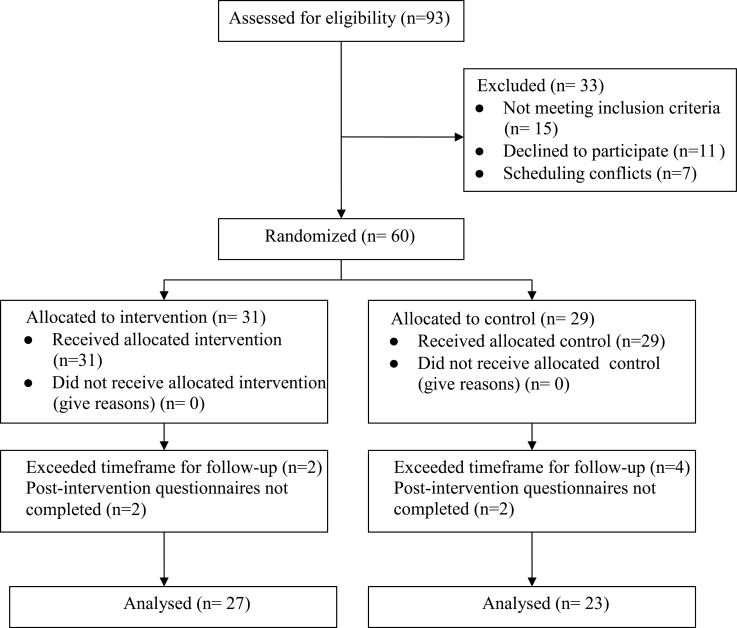
The number of patients referred into the study, eligible for participation, randomally assigned to treatment groups, and assessed for outcomes are shown in Figure 1. A total of 93 individuals from two breast cancer clinics were referred. Fifteen patients were ineligible because they did not receive chemotherapy; seven did not consent due to scheduling conflicts. Eleven patients declined to participate due to lack of time, lack of interest, and/or overwhelmed emotional state. Thus, of those eligible and contacted about participation, 84.5% (60/71) agreed to participate in this study.
Figure 1.
CONSORT participant flow chart.
Of the 60 individuals who consented to participate, 10 were excluded from the analysis because postintervention questionnaires were either not completed (n = 7) or completed past a 1-month posttreatment timeline established by the study team (n = 3). Participant characteristics by treatment arm are summarized in Table 1. Professional occupation was reported statistically significantly more often by women in the intervention group than by those in the control group (50.0% vs 19.1%); this was the only statistically significantly different demographic characteristic between groups (P = .04). One-third of intervention group patients never used a PDA before study participation.
Table 1.
Participant characteristics by treatment arm*
| Control (N = 23); N (%) | Intervention (N = 27); N (%) | Total (N = 50); N (%) | P† | |
|---|---|---|---|---|
| Demographic variables | ||||
| Age, y, mean (SD) | 52.1 (8.5) | 49.5 (10.7) | 50.7 (9.7) | .358 |
| Race | ||||
| White | 21 (91.3) | 21 (77.8) | 42 (84) | .261 |
| African American | 2 (8.7) | 6 (22.2) | 8 (16.0) | |
| Marital status | ||||
| Married | 17 (73.9) | 21 (77.8) | 38 (76.0) | >.99 |
| Divorced/separated | 2 (8.7) | 3 (11.1) | 5 (10.0) | |
| Single | 3 (13.1) | 3 (11.1) | 6 (12.0) | |
| Widowed | 1 (4.4) | 0 (0) | 1 (2.0) | |
| Education | ||||
| Grade school | 1 (4.4) | 0 (0) | 1 (2.0) | .170 |
| High school | 2 (8.7) | 1 (14.8) | 6 (12.0) | |
| Some college | 5 (21.7) | 5 (18.5) | 10 (20.0) | |
| College graduate | 9 (39.2) | 3 (11.1) | 12 (24.0) | |
| Graduate degree | 3 (13.0) | 9 (33.3) | 12 (24.0) | |
| Employment | ||||
| Full time | 12 (52.2) | 16 (59.3) | 28 (56.0) | .776 |
| Part time | 7 (30.4) | 3 (11.1) | 10 (20.0) | |
| Retired | 2 (8.7) | 4 (14.8) | 6 (12.0) | |
| Disabled | 2 (8.7) | 1 (3.7) | 3 (6.0) | |
| Unemployed | 0 (0) | 3 (11.1) | 3 (6.0) | |
| Occupation | ||||
| Professional | 4 (19.1) | 13 (50.0) | 17 (36.2) | .036 |
| Administration | 5 (23.8) | 2 (7.7) | 3 (6.4) | |
| Assistant manager/clerical | 8 (38.1) | 1 (3.9) | 9 (19.2) | |
| Skilled/semiskilled | 1 (4.8) | 4 (15.4) | 5 (10.7) | |
| Homemaker | 3 (14.3) | 6 (23.1) | 9 (19.2) | |
| Household Income | ||||
| <$20,000 | 3 (13.6) | 3 (12.0) | 6 (12.8) | .986 |
| $20,000–$39,999 | 3 (13.6) | 2 (8.0) | 5 (10.6) | |
| $40,000–$59,999 | 2 (9.1) | 2 (8.0) | 4 (8.5) | |
| $60,000–$79,999 | 5 (22.7) | 6 (24.0) | 11 (23.4) | |
| $80,000–$99,999 | 4 (18.2) | 4 (16.0) | 8 (17.0) | |
| ≥ $100,000 | 5 (22.7) | 8 (32.0) | 13 (27.7) | |
| Computer use ≥1/week | 20 (87.0) | 23 (92.0) | 43 (89.6) | .660 |
| Previous PDA use (yes) | — | 18 (66.7) | — | — |
* SD = standard deviation.
† P values based on a two-sided t test for age; else, Fisher exact two-sided test for categorical variables.
Impact of Intervention on Pain, Depression, and Fatigue
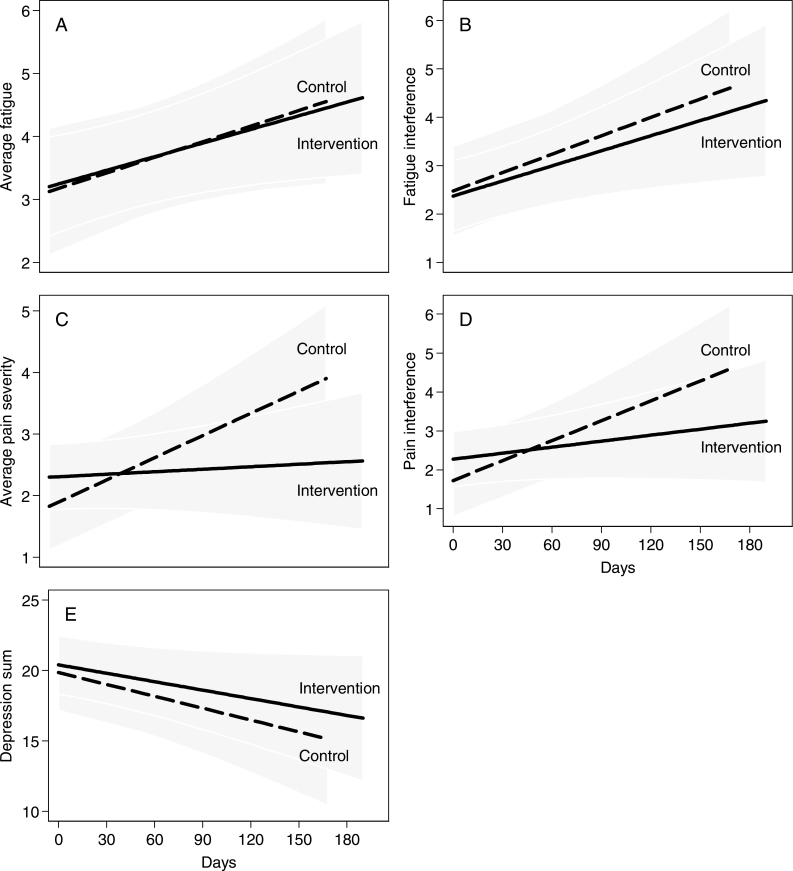
Figure 2 presents graphs that depict changes in average symptom scores by treatment arm over time. No statistically significant differences between groups were found in rates of average fatigue severity (P = .96) or average fatigue interference (P = .78) over time (Figure 2, A and B). There was a statistically significant interaction between treatment arm and time for the rate of average pain severity (P = .02), and a marginally statistically significant interaction for the rate of average pain interference (P = .07) over time (Figure 2, C and D). There was no statistically significant difference between groups in the rate of average depression scores (P = .71) over time (Figure 2, E).
Figure 2.
Patient-reported symptom severity and interference by treatment arm. A) Change from baseline to 160 days of average fatigue severity by treatment arm. B) Change from baseline to 160 days of average fatigue interference by treatment arm. C) Change from baseline to 160 days of average pain severity by treatment arm. D) Change from baseline to 160 days of average pain interference by treatment arm. E) Change from baseline to 160 days of average depression by treatment arm.
Table 2 shows the changes in symptoms across the intervention and control groups after 160 days of study participation. For control group participants, changes over time were statistically significant for fatigue interference (+2.03, P = .01), average pain severity (+1.98, P < .01), pain interference (+2.73, P < .01) and depression (−4.50, P = .04). For intervention group participants, change over time was statistically significant for average fatigue (+1.19, P = .03) and fatigue interference (+1.66, P = .01). For all participants, fatigue and pain increased over time, whereas depression decreased.
Table 2.
Change in symptoms across intervention and control groups after 160 days of participation*
| Symptom | Day 160: intervention − control (95% CI) | P | Control: day 160 − baseline (95% CI) | P | Intervention: day 160 − baseline (95% CI) | P |
|---|---|---|---|---|---|---|
| Average fatigue | −0.09 (−1.73 to 1.55) | .911 | 1.36 (−0.04 to 2.76) | .056 | 1.19 (0.10 to 2.27) | .033 |
| Fatigue interference | −0.47 (−2.51 to 1.57) | .651 | 2.03 (0.45 to 3.61) | .012 | 1.66 (0.34 to 2.98) | .014 |
| Average pain severity | −1.28 (−2.79 to 0.22) | .095 | 1.98 (0.88 to 3.08) | <.001 | 0.22 (−0.62 to 1.06) | .604 |
| Pain interference | −1.36 (−3.44 to 0.73) | .201 | 2.73 (1.20 to 4.25) | <.001 | 0.82 (−0.40 to 2.05) | .188 |
| Depression summary | 1.85 (−4.13 to 7.84) | .544 | −4.50 (−8.72 to −0.28) | .037 | −3.19 (−6.51 to 0.12) | .059 |
* CI = confidence interval.
In addition, results indicated statistically nonsignificant differences for symptom severity outcomes between participants who had used vs those who had not used a PDA before study participation (P = .73 for pain; P = .84 for fatigue; and P = .62 for depression).
HRQoL and Self-Efficacy
Table 3 presents scores on the SF-36 and CASE–Cancer measures. Generally, all participants’ HRQoL scores decreased over the course of chemotherapy treatments. However, the mean pre–post decrease was generally greater among participants in the intervention group, compared with that in control. Pre–post scores on communication self-efficacy were equivalent between groups.
Table 3.
SF-36 and CASE–Cancer scores by treatment arm*
| Health item | Control (N = 23) | Intervention (N = 27) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Pre–post difference | Mean | SD | Pre–post difference | ||
| Physical functioning | Pre | 79.6 | 16.2 | −16.8 | 75.1 | 26.1 | −19 |
| Post | 62.8 | 23.8 | 56.1 | 32.5 | |||
| Physical role | Pre | 16.3 | 27.8 | +6.9 | 38 | 40.1 | −13.9 |
| Post | 23.2 | 37.7 | 24.1 | 39.5 | |||
| Emotional role | Pre | 47.8 | 44.8 | +13.1 | 70.4 | 42.7 | −14.8 |
| Post | 60.9 | 43.4 | 55.6 | 46.2 | |||
| Vitality | Pre | 48.7 | 21.6 | −8.2 | 56.1 | 20.2 | −21.1 |
| Post | 40.5 | 24.4 | 35 | 22.1 | |||
| Mental health | Pre | 66.8 | 21 | +5.8 | 68.5 | 14.2 | −1.4 |
| Post | 72.6 | 17.1 | 67.1 | 14.2 | |||
| Social functioning | Pre | 76.1 | 21 | −11.4 | 73.1 | 24.9 | −21.2 |
| Post | 64.7 | 24.3 | 51.9 | 25.2 | |||
| Bodily pain | Pre | 54.5 | 22.3 | −2.8 | 63.4 | 29.2 | −11.6 |
| Post | 51.7 | 28.1 | 51.8 | 28.9 | |||
| General health | Pre | 56.2 | 19.2 | −7.1 | 57 | 20.7 | −9.9 |
| Post | 49.1 | 20.1 | 47.1 | 23.5 | |||
| CASE–cancer score | Pre | 45 | 4.4 | +0.3 | 44.1 | 3.9 | −0.5 |
| Post | 45.3 | 2.9 | 43.6 | 4.7 | |||
* CASE = Communication and Attitudinal Self-Efficacy; SD = standard deviation; SF = short form.
Feasibility
During usability testing, both patients and clinicians reported that the PDA was easy to use, read, and comprehend (patient mean = 4.63±0.38, clinician mean = 4.60±0.40). Adherence to treatment protocol instructions was excellent, with 83% of the intervention group participants completing symptom inventories and 90% playing videos as instructed. Specifically, the rate of playing videos before each treatment visit was 73% for depression, 86% for fatigue, and 116% for the pain videos. The latter rate of greater than 100% indicates that patients played pain videos more frequently than instructed. Forgetting to complete a study task was the primary reason for nonadherence. Technical difficulties were minimal (0.03%).
Focus Group and Interview Results
Five themes emerged from focus groups/interviews with intervention group patients regarding the effects of study participation. They reported that study participation 1) forced participants to focus on symptoms they were experiencing; 2) allowed them to keep symptoms in perspective; 3) facilitated many opportunities for self-evaluation; 4) validated symptoms that participants experienced as a result of chemotherapy treatments; and 5) helped participants improve their communication with health-care providers.
Clinician Survey Results
Four physicians and four nurse practitioners participated in the study, and 87% (7/8) completed a postintervention survey. The most favorably rated intervention component was the symptom graph provided to clinicians (4.39±0.52). Symptom monitoring (4.07±0.73) and communication videos (3.85±0.85) were also rated positively. The communication tool was highly recommended for future patients (4.43±0.54), and clinicians reportedly valued study participation by their patients (4.24±0.46).
Discussion
This pilot randomized controlled trial tested the effects of a patient-focused communication intervention on pain, depression, and fatigue symptoms among breast cancer patients undergoing chemotherapy. Our study yielded mixed results. Regarding symptom severity/interference, we found a statistically significant intervention effect on pain, but statistically nonsignificant effects on fatigue and depression. Overall, patients played communication videos for pain 30% more frequently than fatigue videos and 43% more often than depression videos. Differences in the intervention dosage that patients received may have been the primary contributor to the positive finding on pain.
Other factors may also have contributed to symptom findings. We measured patients’ perceptions of their own communication behavior via the CASE–Cancer questionnaire, focus groups, and interviews. Focus groups/interviews indicated positive intervention effects on patient communication. CASE–Cancer results were equivocal, probably due to ceiling effects associated with typically high self-ratings of patients’ communication skills (40). Measuring patient perception of communication is valuable but does not fully capture the communication process.
Direct observation (eg, videotaping or audiotaping encounters) has been used to determine patients’ PACE (Presenting information, Asking questions, Checking understanding, Expressing concerns)-concordant communication (41–43,44,45). In our study, perhaps direct observation would have shown that intervention group participants were more effective communicators compared with those in the control group when discussing pain, compared with discussing fatigue and depression. We did not use this methodology in our pilot study due to cost constraints, but it is an important consideration for future studies.
In addition, physician communication plays a statistically significant role in treatment-related outcomes and can also be measured by direct observation and/or perceptual methods (46,47). Although we did not survey patients regarding physicians’ communication behavior, it is conceivable that physicians were more patient-centered during pain-related discussions, compared with discussions on fatigue and depression. Although not measured, it seems likely that physician communication played a role in our findings. Future studies would benefit from the use of observational and perceptual measures of communication by both patients and clinicians. Our future research needs to thoughtfully select additional measures that can help elucidate the pathways connecting intervention to health outcomes (48,49).
In general, HRQoL typically decreases during chemotherapy (50–52). Although the study was not powered on this outcome, it was striking to us that intervention group patients generally perceived a lower average HRQoL. They may have become increasingly aware of problematic symptoms as the study progressed, and this may have contributed to lower HRQoL scores (52). At the same time, however, themes from focus groups and interviews with this group suggested that study participation improved communication, facilitated self-evaluation, validated symptoms, and offered perspective—all factors that might be associated with improvements in HRQoL. These contrasting results suggest the need for further investigation of HRQoL in future studies.
We used a relatively low-tech, noninteractive technology. It allowed us to package an integrated intervention into a mobile device for use in patients’ home environment and facilitated use of a tailored vs “one size fits all” approach. Usability testing suggested that the PDA was easy to use, and feasibility results indicated that it was used at a high rate. Use of technology as a tool to teach patients communication skills is novel and raises questions that need further investigation, including whether or not a technology is warranted and the type of technology that is optimal.
For example, several focus group participants favored the use of an interactive device, such as a smart phone. Advantages of interactivity include immediate transmission of symptom data to clinics and potential involvement of clinic nurses who could review patients’ symptom status and contact patients via technology when a problematic status is indicated. However, this approach could also pose difficulties for patients who are not technology savvy or those without technology access. Although technology-based interventions have successfully addressed digital divide issues, vigilance needs to be exercised regarding potential exacerbation of existing health disparities (53,54).
Conclusion, Limitations, and Future Directions
Our study had several strengths and limitations. First, as the authors are unaware of other studies that investigated a technology-based symptom monitoring and patient communication tool, our pilot findings make an important contribution. Other strengths include the randomized design and use of a tailored, longitudinal approach to communication training. Study limitations include the small sample size, limited generalizability typical of pilot studies and, as previously described, the insufficient measurement of communication and other variables relevant to intervention–outcome relationships.
Future studies should consider an application of the CHAT intervention for a larger and more diverse sample of patients. An application for devices owned by patients might be more favorably viewed and also more cost effective, compared with our method of purchasing, programming, and providing personal digital assistants for the study sample. In addition, research efforts can be expanded to include patient populations at particular risk for higher symptom burden, such as older adults, diverse patient populations (both English and non-English speaking), and patients with comorbidities and additional symptoms. We believe our intervention is transferable to these groups.
Helping cancer patients to effectively communicate symptoms by using information technology can be an important step toward creating a cancer care environment that is tailored, strategic, and participatory (17,55).
Funding
National Cancer Institute (CA115388-02, P30 CA016058 to OSU Compre- hensive Cancer Center Behavioral Measurement Shared Resource, and P50 CA105632 to JLK).
Supplementary Material
The authors are very grateful to the patients who participated in our study and to the clinical staff who supported this research. The content of this work is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute.
References
- 1. American Cancer Society Cancer Facts and Figures, 2004. Atlanta, GA: American Cancer Society, 2004 [Google Scholar]
- 2. Symptom management in cancer: pain, depression, and fatigue. NIH Consens State Sci Statements. 2002;19(4):1–29 [PubMed] [Google Scholar]
- 3. Carr D, Goudas L, Lawrence D, et al. Management of Cancer Symptoms: Pain, Depression, and Fatigue. Evidence Report/Technology Assessment No. 61. Rockville, MD: Agency for Healthcare Research and Quality; 2002. AHRQ Publication No. 02-E032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Jong N, Coutens AM, Abu-Saad HH, et al. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nursing 2002; 25(4):283–297 [DOI] [PubMed] [Google Scholar]
- 5. Jacobsen PB, Meade CD, Stein KD, et al. Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. J Clin Oncology 2002;20(12):2851–2862 [DOI] [PubMed] [Google Scholar]
- 6. Miranda CR, De Resende CN, Melo CF, et al. Depression before and after uterine cervix and breast cancer neoadjuvant chemotherapy. Intl J Genecol Cancer 2002;12(6):773–776 [DOI] [PubMed] [Google Scholar]
- 7. Macquart-Moulin G, Viens P, Bouscary ML, et al. Discordance between physicians’ estimations and breast cancer patients’ self-assessment of side-effects of chemotherapy: an issue for quality of care. Br J Cancer. 1997;76(12):1640–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miaskowski C, Dibble SL. The problem of pain in outpatients with breast cancer. Oncol Nurs Forum. 1995;22(5):791–797 [PubMed] [Google Scholar]
- 9. Kreps GJ. The impact of communication on cancer risk, incidence, morbidity, mortality, and quality of life. Health Commun. 2003;15(2):161–169 [DOI] [PubMed] [Google Scholar]
- 10. Bakker DA, Fitch MI, Gray R, et al. Patient-health care communication during chemotherapy treatment: the perspectives of women with breast cancer. Patient Educ Couns 2001;43(1):61–71 [DOI] [PubMed] [Google Scholar]
- 11. McWilliam CL, Brown JB, Stewart M. Breast cancer patients’ experiences of patient-doctor communication: a working relationship. Patient Educ Couns. 2000;39(2-3):191–204 [DOI] [PubMed] [Google Scholar]
- 12. Ravdin PM, Siminoff IA, Harvey JA. Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol. 1998;16(2):515–521 [DOI] [PubMed] [Google Scholar]
- 13. Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7(11):903–909 [DOI] [PubMed] [Google Scholar]
- 14. Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCann L, Maguire R, Miller M, Kearney N. Patients’ perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care (Engl). 2009;18(2):156–164 [DOI] [PubMed] [Google Scholar]
- 16. Ruland CM, Andersen T, Jeneson A, et al. Effects of an internet support system to assist cancer patients in reducing symptom distress. Cancer Nurs. 2013;36(1):6–17 [DOI] [PubMed] [Google Scholar]
- 17. Hesse BW, Hanna C, Massett HA, et al. Outside the box: will information technology be a viable intervention to improve the quality of cancer care? J Nat Cancer Inst Monogr. 2010;40:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feuerstein M, Ganz PA, eds. Health Services for Cancer Survivors. New York, NY: Springer; 2011 [Google Scholar]
- 19. McGee MR, Gray P. A handheld chemotherapy symptom management system: results from a preliminary outpatient field trial. Health Info J 2005;11:243–258 [Google Scholar]
- 20. Matthew AG, Currie KL, Irvine J, et al. Serial personal digital assistant data capture of health-related quality of life: a randomized controlled trial in a prostate cancer clinic. Health Qual Life Outcomes. 2007;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibson F, Aldiss S, Taylor RM, et al. Utilization of the Medical Research Council evaluation framework in the development of technology for symptom management: the ASyMS-YG Study. Cancer Nurs. 2010;33(5):343–352 [DOI] [PubMed] [Google Scholar]
- 22. Arnold CL, Coran JJ, Hagen MG. Revisiting patient communication training: an updated needs assessment and the AGENDA model. Patient Educ Couns. 2012;88(3):399–405 [DOI] [PubMed] [Google Scholar]
- 23. Epstein RM, Street RL., Jr Patient Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. Bethesda, MD: National Cancer Institute, National Institutes of Health; 2007. NIH Publication No. 07-6225. [Google Scholar]
- 24. Post DM, Cegala DJ, Miser WF. The other half of the whole: teaching patients to communicate with physicians. Fam Med. 2002;34(5):344–352 [PubMed] [Google Scholar]
- 25. Kofoed S, Breen S, Gough K, et al. Benefits of remote real-time side-effect monitoring systems for patients receiving cancer treatment. Oncol Rev. 2012;6(1):51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith JC, Schatz BR. Feasibility of mobile phone-based management of chronic illness. AMIA Annu Symp Proc. 2010;2010:757–761 [PMC free article] [PubMed] [Google Scholar]
- 27. Haque M, Kawsar F, Adibuzzman M, et al. Findings of e-ESAS: a mobile based symptom monitoring system for breast cancer patients in rural Bangladesh. In: Conference on Human Factors in Computing Systems; Austin, TX: Special Interest Group on Computer-Human Interaction; 2012: 899–908 [Google Scholar]
- 28. Cleeland CS. Measurement of pain by self-report. In: Chapman CR, Loeser JD. eds. Advances in Pain Research and Therapy. New York, NY: Raven Press; 1989:391–403 [Google Scholar]
- 29. Mendoza T, Mayne T, Rublee D, et al. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10(4):353–361 [DOI] [PubMed] [Google Scholar]
- 30. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401 [Google Scholar]
- 31. Hann DK, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the center for epidemiological studies depression scale (CES-D). J Psychosomatic Res. 1999;46(5):437––443 [DOI] [PubMed] [Google Scholar]
- 32. Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–310 [DOI] [PubMed] [Google Scholar]
- 33. Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9(7):847–854 [DOI] [PubMed] [Google Scholar]
- 34. Naughton MJ, Wiklund I. A critical review of dimension-specific measures of health-related quality of life in cross-cultural research. Qual Life Res. 1993;2(6):397–432 [DOI] [PubMed] [Google Scholar]
- 35. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483 [PubMed] [Google Scholar]
- 36. Ware JE, Jr, Kosinki M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994 [Google Scholar]
- 37. Wolf MS, Change CH, Davis T, et al. Development and validation of the Communication and Attitudinal Self- Efficacy Scale for cancer (CASE–cancer). Patient Educ Couns. 2005;57(3):333–341 [DOI] [PubMed] [Google Scholar]
- 38. Clayman ML, Pandit AU, Bergeron AR, et al. Ask, understand, remember: a brief measure of patient communication self-efficacy within clinical encounters. J Health Comm. 2010; 15(suppl 2):72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frison L, Pocock S. Repeated measures in clinical trial: analysis using mean summary statistics and its implication for design. Statistics Med. 1993;11(13):1685–1704 [DOI] [PubMed] [Google Scholar]
- 40. Cegala DJ, Coleman MT, Turner JW. The development and partial assessment of the medical communication competence scale. Health Commun. 1998;10(3):261–288 [DOI] [PubMed] [Google Scholar]
- 41. Cegala DJ, Post DM. The impact of patients’ participation on physicians’ patient-centered communication. Patient Educ Couns. 2009;77(2):202–208 [DOI] [PubMed] [Google Scholar]
- 42. Post DM, Cegala DJ, Marinelli T. Teaching patients to effectively communicate with physicians: the impact of race. J Natl Med Assoc. 2001;93(1):6–12 [PMC free article] [PubMed] [Google Scholar]
- 43. Cegala DJ, Marinelli T, Post DM. The effects of patient skills training on compliance. Arch Fam Med. 2000;9(1):57–64 [DOI] [PubMed] [Google Scholar]
- 44. Cegala DJ, Post DM, McClure L. The effects of patient communication skills training on the discourse of older patients during a primary care interview. J Am Geriatr Soc. 2001;49(11):1505–1511 [DOI] [PubMed] [Google Scholar]
- 45. Cegala DJ, McClure L, Marinelli TM, Post DM. The effects of communication skills training on patients’ participation during medical interviews. Patient Educ Couns. 2000;41(2):209–222 [DOI] [PubMed] [Google Scholar]
- 46. Arora NK. Interacting with cancer patients: the significance of physicians’ communication behavior. Soc Sci Med. 2003;57(5):791–806 [DOI] [PubMed] [Google Scholar]
- 47. Epstein RM, Franks P, Fiscella K, et al. Measuring patient-centered communication in patient-physician consultations: theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528 [DOI] [PubMed] [Google Scholar]
- 48. Street RL, Makoul G, Arora N, et al. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295–301 [DOI] [PubMed] [Google Scholar]
- 49. Epstein RM. Making communication matter: what do patients notice, what do patients want, and what do patients need? Patient Educ Couns. 2006;60(3):272–278 [DOI] [PubMed] [Google Scholar]
- 50. Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention. JAMA. 2006;295(23):2742–2751 [DOI] [PubMed] [Google Scholar]
- 51. Martín M, Lluch A, Seguí MA, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol. 2006;17(8):1205–1212 [DOI] [PubMed] [Google Scholar]
- 52. Browall MM, Ahlberg KM, Persson LO, et al. The impact of age on health-related quality of life (HRQoL) and symptoms among postmenopausal women with breast cancer receiving adjuvant chemotherapy. Acta Oncol. 2008;47(2):207–215 [DOI] [PubMed] [Google Scholar]
- 53. Gustafson DH, McTavish FM, Stengle W, et al. Reducing the digital divide for low-income women with breast cancer: a feasibility study of a population-based intervention. J Health Commun. 2005;10(suppl 1):173–193 [DOI] [PubMed] [Google Scholar]
- 54. Gustafson DH, McTavish FM, Stengle W, et al. Use and impact of eHealth system by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):195–218 [DOI] [PubMed] [Google Scholar]
- 55. Grimsbo GH, Engelsrud GH, Ruland CM, et al. Cancer patients’ experiences of using an Interactive Health Communication Application (IHCA) [published online ahead of print May 9, 2012]. Int J Qualitative Stud Health Well-being.10.3402/qhw.v7i0.15511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.