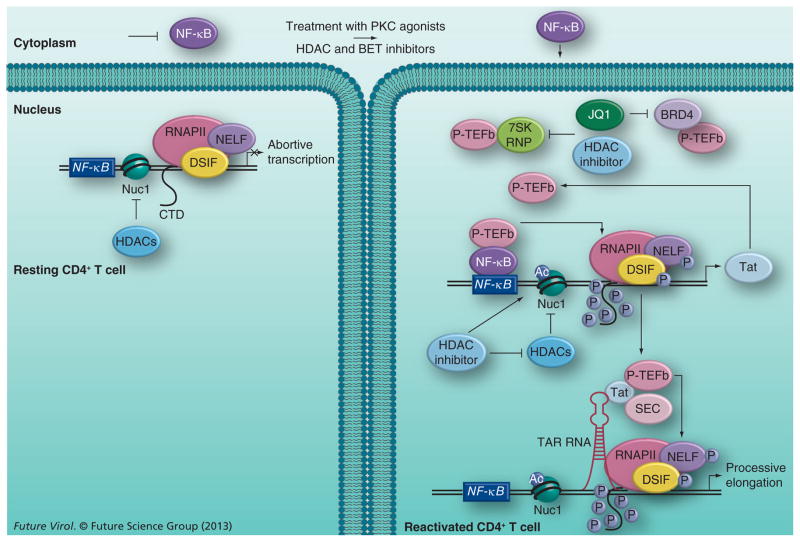
Figure 1. Speculative model of HIV reactivation from latency using histone deacetylase or bromodomain and extraterminal inhibitors.
In resting CD4+ T cells, viral transcription is inhibited due to sequestration of NF-κB (and NFAT, not shown) in the cytoplasm and limiting levels of P-TEFb. The action of HDACs maintains the repressive Nuc1 structure formed at the viral long terminal repeat by deacetylating the histone tails. Upon the addition of PKC agonists and HDAC or BET inhibitors, latent viral reactivation likely occurs in a biphasic process. PKC agonists activate NF-κB, which, upon translocation into the nucleus, binds to the NF-κB site in the viral long terminal repeat and recruits P-TEFb, in a Tat-independent manner, to activate RNAPII transcription of the viral protein Tat. To perpetuate viral transcription, the newly synthesized Tat protein recruits P-TEFb and SEC to the TAR RNA element formed at the 5′ end of viral transcripts. P-TEFb phosphorylates the CTD of RNAPII and the negative elongation factors associated with it to stimulate processive transcription of the viral genome. HDAC and BET inhibitors augment the effect of PKC agonists by freeing up P-TEFb from different cellular reserves and making it available for Tat transactivation. While both inhibitors free it from the 7SK RNP complex through undefined mechanisms, BET inhibitors also release P-TEFb from the BRD4 complex by inhibiting the activity of the latter. The HDAC inhibitors also increase acetylation of the Nuc1 nucleosome by inhibiting the activity of HDACs, which contributes to reversing viral latency.
Ac: Acetylated; BET: Bromodomain and extraterminal; CTD: C-terminal domain; HDAC: Histone deacetylase; P: Phosphorylated; SEC: Super elongation complex.