Fig. 3.
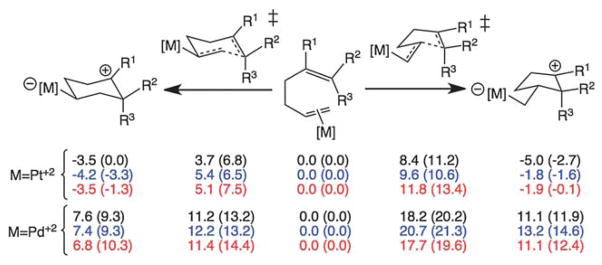
Computed reaction enthalpies (free energies in parentheses; kcal mol−1; in DCE) and barriers for [Pt(PH3)3]2+ and [PdCl2(NCMe)] promoted 6-endo and 5- exo cyclizations. Black are for R1 = R2 = R3 = CH3; blue are for R1 = R3 = CH3/R2 = H; red are for R1 = R2 = CH3/R3 = H. Preferences for 6-endo cyclization with [Pt(PH3)3]2+ are also predicted for systems with a methyl group added to the terminal carbon of the Pt-complexed C=C π-bond (and R1 = R2 = R3 = H or R1 = R2 = R3 = CH3); see ESI† for details.
