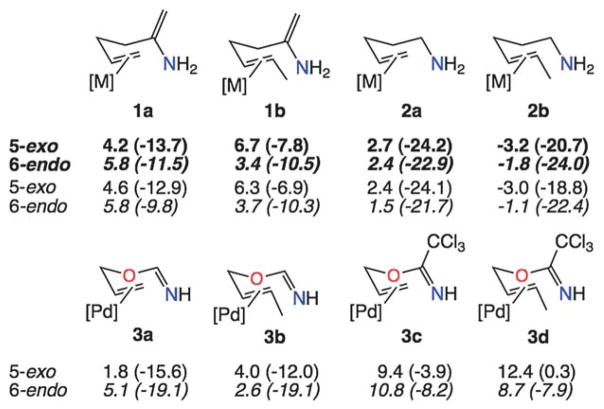
Fig. 4.
Free energy barriers and ender/exergonicities (kcal mol−1; in DCE; exergonicities in parentheses) for systems with nitrogen nucleophiles. For 1a/b and 2a/b, bold values are for [M] = [Pt(PH3)3]2+ and plain text values are for [M] = [Pd(PH3)3]2+. For 3a–d, [M] = [PdCl2(NCMe)]. Barriers for 2b are negative because they are based on an extended rather than productive conformation of the reactant.