Abstract
AIM: To study the association between the incidence of gastric cancer and populational exposure to risk/protective factors through an analysis of international databases.
METHODS: Open-access global databases concerning the incidence of gastric cancer and its risk/protective factors were identified through an extensive search on the Web. As its distribution was neither normal nor symmetric, the cancer incidence of each country was categorized according to ranges of percentile distribution. The association of each risk/protective factor with exposure was measured between the extreme ranges of the incidence of gastric cancer (under the 25th percentile and above the 75th percentile) by the use of the Mann-Whitney test, considering a significance level of 0.05.
RESULTS: A variable amount of data omission was observed among all of the factors under study. A weak or nonexistent correlation between the incidence of gastric cancer and the study variables was shown by a visual analysis of scatterplot dispersion. In contrast, an analysis of categorized incidence revealed that the countries with the highest human development index (HDI) values had the highest rates of obesity in males and the highest consumption of alcohol, tobacco, fruits, vegetables and meat, which were associated with higher incidences of gastric cancer. There was no significant difference for the risk factors of obesity in females and fish consumption.
CONCLUSION: Higher HDI values, coupled with a higher prevalence of male obesity and a higher per capita consumption of alcohol, tobacco, fruits, vegetables and meat, are associated with a higher incidence of gastric cancer based on an analysis of populational global data.
Keywords: Gastric cancer, Risk factors, Epidemiologic factors, Environment, Public health
Core tip: An ecological study on gastric cancer based on public databases proved to be feasible and promising, and this method can be used to monitor the behavior of the disease globally. The results of this study indicated a higher level of development, coupled with the highest prevalence of male obesity and a higher per capita consumption of alcohol, tobacco, fruits, vegetables and meat, among the countries with the highest incidences of gastric cancer. In contrast, a high consumption of vegetables was associated with a lower disease incidence in other countries.
INTRODUCTION
Although its incidence has been declining in many countries, gastric cancer is still the second leading cause of death from malignancy worldwide, accounting for 700349 deaths in 2002[1].
The identification of risk factors associated with malignancies is of great importance because this knowledge can facilitate not only the development of policies aimed at the prevention of cancer occurrence but also the vigilance of at-risk groups regarding the early identification of new cases. Such identification is usually performed through observational studies, and especially case-control and cohort studies, which involve high-cost processes from a financial perspective and a long time to completion. However, those types of studies, although numerous, are usually conducted on limited population sizes, social profiles and geographic locations, with a consequent limitation of the generalizability of the results.
The systematic recording of the health data, social characteristics and habits of living populations and the consequent creation and maintenance of public databases have been made possible by the development of techniques for ecological study. These methods, by definition, allow a shift of the focus of analysis from the individual to the population. Ecological studies allow inferences about the effect of risk conditions on rates of diseases in populations. Despite limitations in measuring individual aspects and in the analysis of the cumulative effects of factors, such studies have great value due to their simplicity and low cost of analysis, may contribute to the development of health policies and may guide research efforts specific to large populations.
Given the notable differences in the frequency and distribution of gastric cancer in the various countries of the world and the operational difficulties of running traditional epidemiological studies on global demographic trends and risk factors, we performed a study on risk factors’ association with gastric cancer through populational database analysis.
MATERIALS AND METHODS
Databases
The age-standardized incidences of stomach cancer in 184 countries were obtained from the public database maintained by the GLOBOCAN Project of the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO), considering the most recent data available (from 2008).
GLOBOCAN project
Over the past 30 years, the IARC has published regular estimates of the incidence of and mortality from major types of cancer in several regions of the world at the national level, focusing on 184 countries, using new sources of data and improved methods of estimation. The results of the data collection in 2008 can be summarized as follows: (1) National incidence data - systematically collected by IARC member countries (62 countries); (2) Data from one or multiple local records - presented by the weighted average (75 countries); (3) Data derived from known frequencies of all types of cancer - adjusted for the known relative frequency of each type (13 countries); and (4) No data collected - presents data from neighboring countries in the same geographic region (34 countries).
Estimates are presented separately for each sex and divided into 10 age groups. These values are based on the most recent data available at the IARC and on publicly available information on the Internet, including data from the cancer registries of populational databases. These databases can cover an entire national population but more often cover smaller, subnational areas, and in developing countries, only large cities.
Furthermore, the degree of delay was taken into account by computing predictions. Although historical trends may not continue in the future, predictions based on linear trend patterns have been empirically shown to be reasonably accurate, particularly in the short term.
When historical data and a sufficient number of registered cases were available, the incidence rates were projected for 2008. Otherwise, the incidence rates of the most recent period were applied.
Regional models were used when data on the incidence in specific countries or locations were absent or when the data were considered to be of insufficient quality. In the absence of data, the values of neighboring countries in the same region were used.
Risk and protective factors
For the selection of risk and protective factors described in the medical literature, an extensive review was conducted through the search engines MEDLINE and PubMed, using the following keywords and limiters: gastric cancer, risk factors, etiology, epidemiology and diet factors. After characterizing these factors, we performed a search of specific databases to determine populational exposure to the factors using the Google search engine, with emphasis on references from government agencies and nongovernmental organizations linked to each marker. The year of reference for each factor was 2008 or the closest available year.
Helicobacter pylori
We analyzed data from studies published between 1990 and 2012 in PubMed and MEDLINE using the following key words and limiters: Helicobacter pylori (H. pylori), Helicobacter, Helicobacter pylori, incidence, prevalence and epidemiology. We performed this search because there is no global database containing the values of the incidence and prevalence of colonization by the agent. The values collected showed a lack of precision and a large omission of data, as we could identify references to only 54 of the 183 studied countries. Thus, we decided to exclude this risk factor from further analysis, despite its clinical relevance.
Tobacco
Data were collected from the Global Health Observatory Data Repository of the WHO. The reference variable used was the percentage of the population using any tobacco product (age-standardized rate).
Alcohol
Data were collected from the World Health Statistics database of the Global Health Observatory Data Repository of the WHO. The reference variable used was liters of pure alcohol/person/year.
Obesity
Data were collected from the World Health Statistics database of the Global Health Observatory Data Repository of the WHO. The reference variable used was the percentage of adults over 20 years that present a body mass index (BMI) > 30 kg/m2.
Consumption of fruits, vegetables, legumes, meat and fish
Data were collected using the FAOSTAT tool of the Food and Agriculture Organization (FAO) database of the United Nations. The reference variable used was consumption in g/person/d.
Salt consumption
We could not find databases regarding average per capita salt intake or consumption in different countries, so this factor was excluded from our analysis.
Human development index
Data on the human development index (HDI) were collected from the database of Human Development Reports of the United Nations.
Statistical analysis
The bivariate relationship of characteristics has been primarily studied by plotting data on the incidence of gastric cancer and various numerical indicators associated with risk in scatter plots. As the incidence of gastric cancer in 183 countries did not have a normal (Kolmogorov-Smirnov P < 0.001) or symmetric (histogram analysis) distribution, the distribution of incidences was categorized into percentile ranges (10, 25, 50, 75 and 90). A new graphical analysis of the association between the incidence of cancer (categorized) and measures of exposure to each risk factor was performed using box plots.
The Mann-Whitney test was then used to test for differences in measures of exposure to each risk factor between the extreme ranges of incidence (under the 25th percentile and above the 75th percentile) (Table 1).
Table 1.
List of countries categorized by extreme incidences
| < P25 | Incidence | > P75 | Incidence |
| Botswana | 0.2 | Chinese Taipei | 15.6 |
| Namibia | 0.7 | Poland | 15.6 |
| Malawi | 0.8 | Singapore | 15.8 |
| Lesotho | 0.9 | Austria | 15.9 |
| Sudan | 1.0 | Bosnia and Herzegovina | 16.2 |
| Swaziland | 1.0 | Azerbaijan | 16.4 |
| Tanzania | 1.0 | Bhutan | 16.6 |
| Central African Republic | 1.1 | Uruguay | 16.7 |
| Chad | 1.1 | Guatemala | 17.0 |
| Eritrea | 1.1 | Honduras | 17.0 |
| Gambia | 1.1 | Slovakia | 17.2 |
| Cameroon | 1.2 | Vietnam | 17.3 |
| Comoros | 1.2 | Spain | 17.5 |
| Equatorial Guinea | 1.2 | Peru | 18.1 |
| Gabon | 1.2 | Georgia | 18.6 |
| Niger | 1.2 | Germany | 18.6 |
| Nigeria | 1.2 | Kyrgyzstan | 18.6 |
| Republic of Congo | 1.3 | Romania | 18.7 |
| Djibouti | 1.3 | Moldavia | 18.8 |
| Gaza Strip | 1.3 | Jamaica | 19.3 |
| Maldives | 1.3 | Hungary | 20.8 |
| Mozambique | 1.5 | Costa Rica | 20.9 |
| Syria | 1.5 | Kazakhstan | 21.4 |
| United Arab Emirates | 1.5 | Armenia | 21.8 |
| Saudi Arabia | 1.7 | Slovenia | 22.3 |
| Egypt | 1.8 | Chile | 22.4 |
| Togo | 1.8 | Ecuador | 22.4 |
| Benin | 1.9 | Croatia | 22.6 |
| Burkina Faso | 1.9 | Mongolia | 22.8 |
| Ethiopia | 1.9 | Macedonia | 22.9 |
| Zambia | 1.9 | Bulgaria | 23.5 |
| Kuwait | 2.0 | Montenegro | 23.6 |
| Qatar | 2.0 | Italy | 26.0 |
| Sri Lanka | 2.0 | Ukraine | 26.2 |
| Iraq | 2.1 | Latvia | 26.8 |
| Yemen | 2.1 | Albania | 26.9 |
| Angola | 2.2 | Portugal | 27.1 |
| Sierra Leone | 2.2 | Lithuania | 27.6 |
| Republic of South Africa | 2.2 | Estonia | 27.9 |
| Ivory Coast | 2.4 | Russia | 28.7 |
| Laos | 2.4 | China | 34.5 |
| Oman | 2.4 | Belarus | 36.4 |
| Somalia | 2.4 | South Korea | 56.3 |
| Solomon Islands | 2.5 | Japan | 80.2 |
| Ghana | 2.6 | ||
| Libya | 2.6 | ||
| Vanuatu | 2.6 |
A database of collected data was created using the software MS Excel, and data analysis was performed using SPSS v. 13.0, considering a significance level of 0.05.
As the research was performed using open-access public databases, it was unnecessary to obtain express authorization from the maintainers of such data. The data’s sources are properly cited along with the disclosure of the results of this study, as recommended by the sources.
We could not identify any conflicts of interest or ethical conflicts in the implementation of the present study, so submission for analysis by the Committee of Ethics in Research was not necessary, according to its own rules.
RESULTS
In the cross-analysis of variables from different databases, which is the basis of this study, it was expected that values for each risk factor under study could not be found for all countries. Thus, the availability of data ranged from 58 countries for the prevalence of H. pylori to 172 countries for the consumption of alcohol and obesity, considering the 183 countries for which the incidence of gastric cancer was available in the GLOBOCAN database.
A visual analysis of the scatter plots of the incidence of gastric cancer and the study variables suggested a weak or nonexistent correlation, as exemplified by Figure 1.
Figure 1.
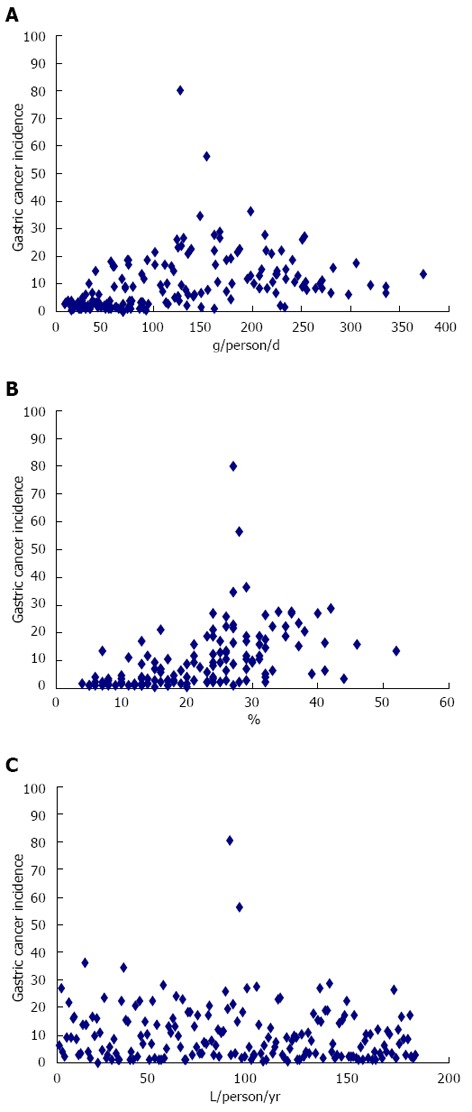
Several of the scatter plots. A: Meat; B: Tobacco; C: Alcohol.
By the selection of extreme incidences of gastric cancer according to percentile distribution [under the 25th percentile (< P25) and above the 75th percentile (> P75)], a different view of the association was possible. The countries categorized in the extreme incidence ranges are presented in the world map in Figure 2.
Figure 2.
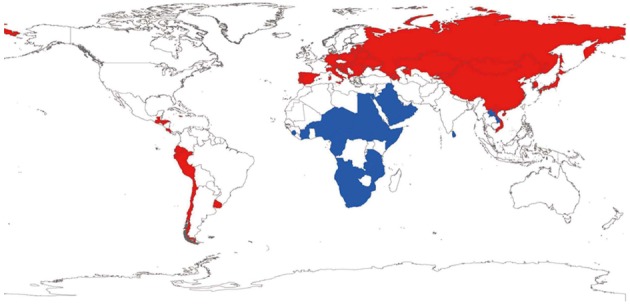
World map of countries with extreme incidences of gastric cancer. Blue: Countries categorized as < P25; Red: Countries categorized as > P75.
The Mann-Whitney test showed differences in measures of certain risk factors between the extreme incidence ranges, as summarized in Table 2.
Table 2.
Association between the incidence of gastric cancer and risk/protection factors based on the Mann-Whitney test
| Risk/protective factors | n | n (< P25) | Average | SD | n (> P75) | Average | SD | P value |
| HDI | 171 | 46 | 0.52 | 0.14 | 43 | 0.76 | 0.09 | 0.000 |
| Alcohol, liters of alcohol/inhabitant per year | 172 | 45 | 3.74 | 3.42 | 43 | 10.59 | 5.17 | 0.000 |
| Tobacco, % of smoking population | 132 | 36 | 14.47 | 7.27 | 35 | 30.03 | 7.11 | 0.000 |
| Fruits, g/person per day | 165 | 42 | 145.84 | 109.74 | 40 | 222.9 | 89.92 | 0.001 |
| Vegetables, g/person per day | 165 | 42 | 154.35 | 151.55 | 40 | 318.66 | 167.25 | 0.000 |
| Legumes, g/person per day | 165 | 42 | 23.08 | 19.88 | 41 | 8.42 | 8.91 | 0.000 |
| Meat, g/person per day | 165 | 42 | 63.26 | 51.07 | 42 | 157.05 | 63.16 | 0.000 |
| Fish, g/person per day | 162 | 42 | 42.56 | 74.83 | 38 | 44.93 | 42.14 | 0.260 |
| Obesity in males, % men > 20 yr and BMI > 30 kg/m2 | 172 | 46 | 9.56 | 102.11 | 42 | 17.03 | 67.73 | 0.000 |
| Obesity in females, % women > 20 yr and BMI > 30 kg/m2 | 172 | 46 | 18.94 | 150.44 | 42 | 21.66 | 81.28 | 0.100 |
HDI: Human development index; BMI: Body mass index.
Countries in the > P75 range of the incidence of gastric cancer had a significantly higher average HDI (0.76019 vs 0.52404) than countries in the < P25 range.
The consumption of alcohol was associated with higher incidences of gastric cancer, with an average consumption of 10.60 L/person/year, which is significantly higher than the consumption in countries with incidences below the 25th percentile (3.75 L/person/year).
The consumption of tobacco was also associated with the highest incidences of gastric cancer, with an average of 30.03% of the population being consumers of any tobacco product, compared with an average of 14.47% among countries with incidences of cancer below the 25th percentile.
A higher consumption of fruits was related to higher incidences of gastric cancer. An average consumption of 222.9 g/d was observed among countries with incidences above the 75th percentile, and consumption of 145.8 g/d was observed among countries with incidences below the 25th percentile.
The same direction of association was found for vegetable consumption, with an average consumption of 318.7 g/d among countries with the highest incidences of gastric cancer and an average of 154.4 g/d among countries below the 25th percentile of the distribution of cancer incidence.
However, a higher consumption of legumes was significantly associated with lower incidences of gastric cancer.
The highest per capita consumption of meat was related to higher incidences of gastric cancer, as an average consumption of 157.1 g/d/person was found in countries grouped above the 75th percentile of the distribution of the incidence of gastric cancer, in comparison with an average of 63.3 g/person/d in countries below the 25th percentile.
In contrast, no significant association was observed for between the average consumption of fish and the population rates of female obesity.
Regarding the national percentage of obese male adults, the difference was significant, as the highest rates of obesity were associated with higher incidences of gastric cancer.
These results were reinforced by the analysis of boxplot diagrams shown in Figure 3.
Figure 3.
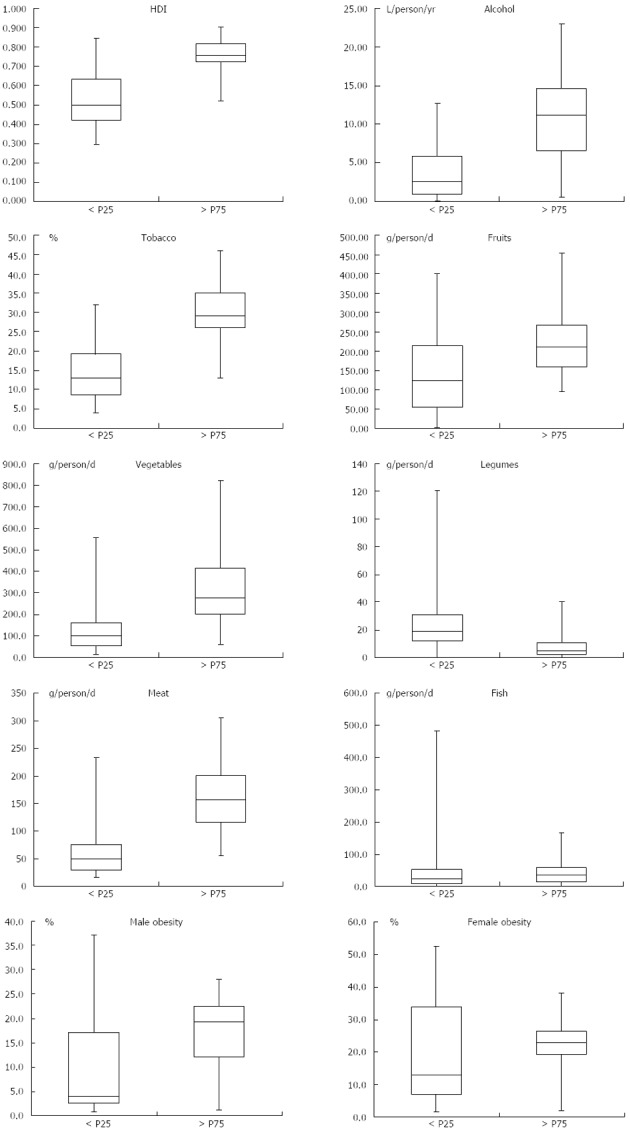
Boxplot diagrams of human development index values. the consumption of alcohol, tobacco, fruits, vegetables, legumes, meat and fish; and the prevalence of obesity in males and females among countries with gastric cancer incidences < P25 and > P75.
DISCUSSION
The prevention and treatment of stomach cancer, which is currently the fourth most common malignancy worldwide, are still major challenges[2-4].
There are geographic and ethnic differences in the distribution of the incidence of gastric cancer worldwide and changing trends in each population over time, which hinder a better understanding of this cancer’s etiology.
It is assumed that the incidence has been decreasing in most industrialized countries over the past three decades and that the incidence patterns observed in immigrant groups move toward the patterns in the countries of origin. These changes suggest a close association of gastric cancer with modifiable factors[5-11].
During the development of this study, when we first analyzed the crude data from all countries, we could not detect significant associations between protective/risk factors and gastric cancer due to the weak correlations observed. However, when studying the extreme incidences, we observed evidence of several of the associations already described in the literature between dietary/behavioral factors and the incidence of gastric cancer.
The IARC has presented fruits and vegetables as probable protective factors in the development of stomach cancer. Therefore, the World Cancer Research Fund recommends a daily intake of vegetables/fruits greater than 400 g for a protective effect. This association was not observed in the current study. Moreover, although gastric cancer is considered to be a multifactorial disease, we observed that several of the countries with higher incidences, such as Korea, had a per capita consumption of fruits and vegetables above the suggested protective intake.
Part of this result may be justified by specific dietary components and certain cooking practices that are also associated with an increased risk of gastric cancer through the formation of N-nitroso compounds and polycyclic aromatic hydrocarbons. These practices include grilling, baking, curing, drying in the sun, smoking, cooking, frying in open ovens and salting. Certain foods also have natural nitrate concentrations (cabbage, cauliflower, carrots, celery, radishes, beets and spinach), or these compounds may be added during preservation. In addition, the nitrate content in soil fertilizers and water also contributes to the level of dietary nitrate[12].
When evaluating obesity in males and the consumption of meat, vegetables, tobacco and alcohol, the results were consistent with findings described in the literature, reinforcing the concept that a modification of lifestyle represents a practical strategy for preventing gastric cancer, especially in middle-aged or elderly people[12-14].
Currently, more than 80% of cases of gastric cancer may be associated with infection by H. pylori. In general, both cancer and infection tend to affect more individuals of lower socioeconomic classes, presumably due to poor education and sanitary conditions[15-21]. Due to the unavailability of a worldwide database representing this factor, we could not study its association with gastric cancer incidence.
It is known that the incidence rates of gastric cancer can vary up to 10 times worldwide and that nearly two-thirds of stomach cancers occur in developing countries. Even so, Japan and Korea, countries with high levels of development, have the highest rates of gastric cancer in the world[22-27].
One of the possible explanations for this finding might be the large difference not only in the etiology but also in the programs for early detection, specialized treatment and prevention in these countries. Furthermore, there is evidence that in areas of low incidence, genetic and biological characteristics seem to have a greater influence on the development of the disease. For example, the incidence of cancer in Africa is the lowest among all developing and developed countries, ranging from 2 to 5.6/100000[5,28].
It is important to remember that proximal tumors are more common in developed countries and in higher socioeconomic classes and have shown a progressive increase in incidence[29]. Distal tumors remain predominant in Japan, the country with the highest incidence, in contrast to the rest of the world[14,29].
The multifactorial etiology of gastric cancer imposes an additional challenge on the understanding and development of effective prevention and monitoring programs. The most appropriate epidemiological studies that are focused on factors associated with the disease require long and careful monitoring because these studies are based on observing individual cases, exposure to factors and cancer development, which makes these studies extremely expensive. The present study, with no claim to challenge epidemiological data or to create a method to replace epidemiological studies, proposes the implementation of a simple, low-cost methodology for the evaluation of the relationship between risk factors and outcome.
Ecological studies are valued in research on seasonal changes or geographical variations of events, especially under adverse social or territorial conditions that make it impossible to study every citizen. However, such studies have limitations. By shifting the focus from the subject to the population, one can lose sight of the direct relationship between a risk/protective factor and individual development of the disease. However, as exposure is measured in an ecological way, it is assumed that an average variation in the incidence of gastric cancer in a particular country also reflects a variation in the average exposure of each individual residing there[30-33]. This concept can be taken into consideration when evaluating individual consumption of food, tobacco products or alcohol.
The quality of a database depends on the properties of the components used in its formulation and the accuracy of the information systems used in its construction, including the database’s integrity (completeness) and internal consistency (data consistent and not contradictory). The systematic application of operational definitions and standardized procedures for measuring and calculating allows inferences about and predictions of unavailable data[30,31].
Although we did not spare efforts to identify the best data needed for our research, the fact that we worked with secondary data implies a limitation of this study, especially given a lack of access to the primary measures. This lack of data disallowed the proper analysis of major risk factors, such as salt intake and exposure to H. pylori.
The attempt to create a database for the prevalence of infection by H. pylori from data from published studies to which we had access resulted in very small number of evaluated countries, which could have affected the final outcome. Thus, we disregarded the results of this analysis in our conclusions. Due to its importance in the genesis of gastric cancer, we consider the creation of a global database for the prevalence of H. pylori infection in each country to be important.
The inaccuracy of cancer incidence data for certain countries due to the methodology used by GLOBOCAN, which is considered to be a relatively reliable and stable source, being widely used in technical and scientific papers, should be noted. However, the collection of data from GLOBOCAN incorporates data measured by national health agencies and data derived from approximations of incidence based on the known frequencies of all types of cancer. In addition, 34 countries do not have local data, leading to the use of data available for neighboring countries in the same geographic region[4].
The data collected from the FAO of the United Nations include global information from national statistical offices with internationally recognized definitions, concepts and classifications. Time series statistics have been compiled, processed and stored by each country since 1961, and the database contains the records of more than 245 countries and territories. The data are provided by governments through national publications and FAO questionnaires (paper or electronic). To make the data coverage as complete as possible, the official data are occasionally supplemented with data from unofficial sources.
The ecological study of gastric cancer based on public databases proved to be feasible and promising, and this method can be used to monitor the global behavior of similar diseases.
ACKNOWLEDGMENTS
Our special thanks to Prof. Dr. Mauro Souza Leite Pinho, coordinator of the Research Group on Cancer Epidemiology of Univille, for his ideas and support.
COMMENTS
Background
The multifactorial etiology of gastric cancer imposes an additional challenge on the understanding and development of effective prevention and monitoring programs. Ecological studies are valued in research on seasonal changes or geographical variations of events, especially under adverse social or territorial conditions that make it impossible to study every citizen.
Research frontiers
The results of ecological studies can provide the opportunity for more carefully designed studies based on the initial observations. The use of databases is a simple, low-cost methodology for the evaluation of the relationship between risk factors and outcome, using data that are generally already available. The quality of a database is crucial to the analysis results.
Innovations and breakthroughs
An ecological study on gastric cancer based on public databases proved to be feasible and promising, and this method can be used to monitor the behavior of the disease globally. The results of this study indicated a higher level of development, coupled with the highest prevalence of male obesity and a higher per capita consumption of alcohol, tobacco, fruits, vegetables, and meat, among the countries with the highest incidences of gastric cancer. In contrast, a high consumption of vegetables was associated with a lower disease incidence in other countries.
Applications
The study’s results suggest that ecological studies using populational databases can be used to monitor the global behavior of the disease. For this purpose, it is important to create a global database for the prevalence of Helicobacter pylori (H. pylori) infection in each country.
Peer review
This ecological study on gastric cancer, based on public databases, provides a useful contribution to the dimension of gastric cancer protection, and the method can be used to monitor the behavior of the disease globally, although the study implies a limitation of using secondary data. The results of this study indicated a higher level of development, coupled with the highest prevalence of male obesity and a higher per capita consumption of alcohol, tobacco, fruits, vegetables and meat, among the countries with the highest incidences of gastric cancer. In contrast, a high consumption of vegetables was associated with a lower disease incidence in other countries. The concern in this study is the exclusion of H. pylori, which is a very important risk factor for gastric cancer, from the analysis.
Footnotes
P- Reviewers: Chuah SK, Dumitrascu DL, Kopacova M, Li YY, Lu XM S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230–236. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Liu H, Wang LE, Wei Q. A functional NQO1 609C& gt; T polymorphism and risk of gastrointestinal cancers: a meta-analysis. PLoS One. 2012;7:e30566. doi: 10.1371/journal.pone.0030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milosavljevic T, Kostic-Milosavljevic M, Jovanovic I, Krstic M. Gastrointestinal and liver tumours and public health in Europe. Eur Rev Med Pharmacol Sci. 2010;14:259–262. [PubMed] [Google Scholar]
- 8.Herszényi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249–258. [PubMed] [Google Scholar]
- 9.Fitzsimmons D, Osmond C, George S, Johnson CD. Trends in stomach and pancreatic cancer incidence and mortality in England and Wales, 1951-2000. Br J Surg. 2007;94:1162–1171. doi: 10.1002/bjs.5751. [DOI] [PubMed] [Google Scholar]
- 10.Anderson WF, Camargo MC, Fraumeni JF, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park B, Shin A, Park SK, Ko KP, Ma SH, Lee EH, Gwack J, Jung EJ, Cho LY, Yang JJ, et al. Ecological study for refrigerator use, salt, vegetable, and fruit intakes, and gastric cancer. Cancer Causes Control. 2011;22:1497–1502. doi: 10.1007/s10552-011-9823-7. [DOI] [PubMed] [Google Scholar]
- 13.Tsugane S. [Primary prevention of gastric cancer] Nihon Rinsho. 2012;70:1720–1725. [PubMed] [Google Scholar]
- 14.Nagel G, Linseisen J, Boshuizen HC, Pera G, Del Giudice G, Westert GP, Bueno-de-Mesquita HB, Allen NE, Key TJ, Numans ME, et al. Socioeconomic position and the risk of gastric and oesophageal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int J Epidemiol. 2007;36:66–76. doi: 10.1093/ije/dyl275. [DOI] [PubMed] [Google Scholar]
- 15.Correa P. Gastric cancer: two epidemics? Dig Dis Sci. 2011;56:1585–156; author reply 1586. doi: 10.1007/s10620-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 16.Correa P, Piazuelo MB. Evolutionary History of the Helicobacter pylori Genome: Implications for Gastric Carcinogenesis. Gut Liver. 2012;6:21–28. doi: 10.5009/gnl.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–498. doi: 10.1038/ajg.2009.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16 Suppl 1:1–9. doi: 10.1111/j.1523-5378.2011.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 21.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham DY, Asaka M. Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J Gastroenterol. 2010;45:1–8. doi: 10.1007/s00535-009-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 24.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–256. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 25.Zilberstein B, Jacob CE, Cecconello I. Gastric cancer trends in epidemiology. Arq Gastroenterol. 2012;49:177–178. doi: 10.1590/s0004-28032012000300001. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto S. Stomach cancer incidence in the world. Jpn J Clin Oncol. 2001;31:471. doi: 10.1093/jjco/31.9.471. [DOI] [PubMed] [Google Scholar]
- 27.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11:135–140. doi: 10.5230/jgc.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, Inoue M. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol. 2011;17:4421–4428. doi: 10.3748/wjg.v17.i39.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 30.Jutte DP, Roos LL, Brownell MD. Administrative record linkage as a tool for public health research. Annu Rev Public Health. 2011;32:91–108. doi: 10.1146/annurev-publhealth-031210-100700. [DOI] [PubMed] [Google Scholar]
- 31.Chen YC, Wu JC, Haschler I, Majeed A, Chen TJ, Wetter T. Academic impact of a public electronic health database: bibliometric analysis of studies using the general practice research database. PLoS One. 2011;6:e21404. doi: 10.1371/journal.pone.0021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Høstgaard AM, Pape-Haugaard L. Reusable data in public health data-bases-problems encountered in Danish Children’s Database. Stud Health Technol Inform. 2012;180:609–613. [PubMed] [Google Scholar]
- 33.Karmel R, Gibson D. Event-based record linkage in health and aged care services data: a methodological innovation. BMC Health Serv Res. 2007;7:154. doi: 10.1186/1472-6963-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]


